The US Food and Drug Administration (FDA) has provided clearance for AMRA Medical’s magnetic resonance (MR) diagnostic software application, AMRA Profiler, for assessment of body composition.
The device can be used for non-invasive fat and muscle evaluation. It allows generation, display and review of MR-based body composition measurements.
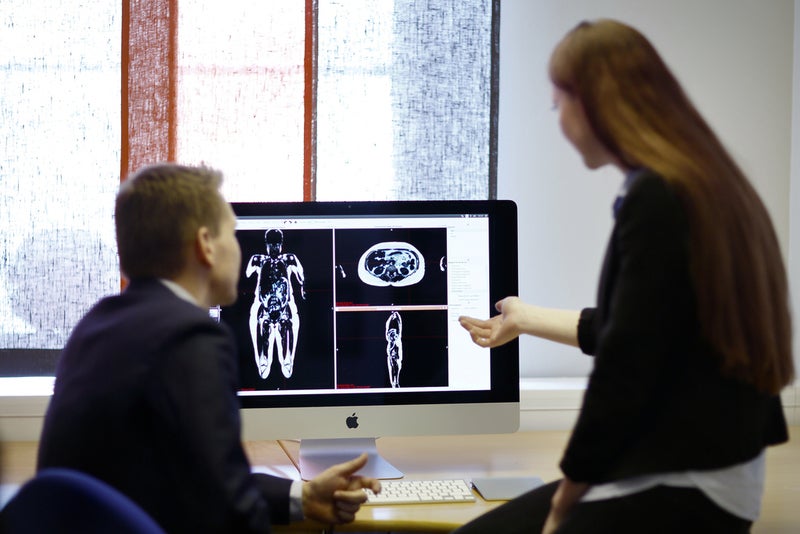
AMRA said that the Profiler transforms MR-images from a six-minute whole-body MRI scan into three-dimensional (3D) volumetric fat and muscle measurements.
It is claimed that this approach facilitates improved accuracy and precision during evaluation of volume and distribution of fat and muscles, and metabolic status.
AMRA CEO Eric Converse said: “AMRA Profiler helps address these challenges by providing physicians with the most detailed body composition assessment and imaging available, cost-effectively and with minimal intrusion to the patient.
“Ultimately this enables clinicians to make more informed treatment decisions about the whole body.”
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataAMRA Profiler enables a variety of body composition measurements, including visceral and subcutaneous adipose tissue volumes, liver fat fraction, thigh muscle volume and related medical images.
Interpretation of these images by a trained physician provides relevant diagnostic information.
The device holds the European CE-Mark and is available for clinical use in Sweden, the UK, and Germany.
Converse added: “This clearance is the next step in our journey of translating the benefits of AMRA into clinical practice and in ultimately contributing to the real world data and real-world evidence that are playing an increasing role in healthcare decisions today.”
According to AMRA, body composition profiling is a combination of variables, certain biomarkers and indices that collectively report fat and muscle distribution.
The company hopes that in future, the method will aid highly targeted drugs and lifestyle interventions to achieve improvements in health.




