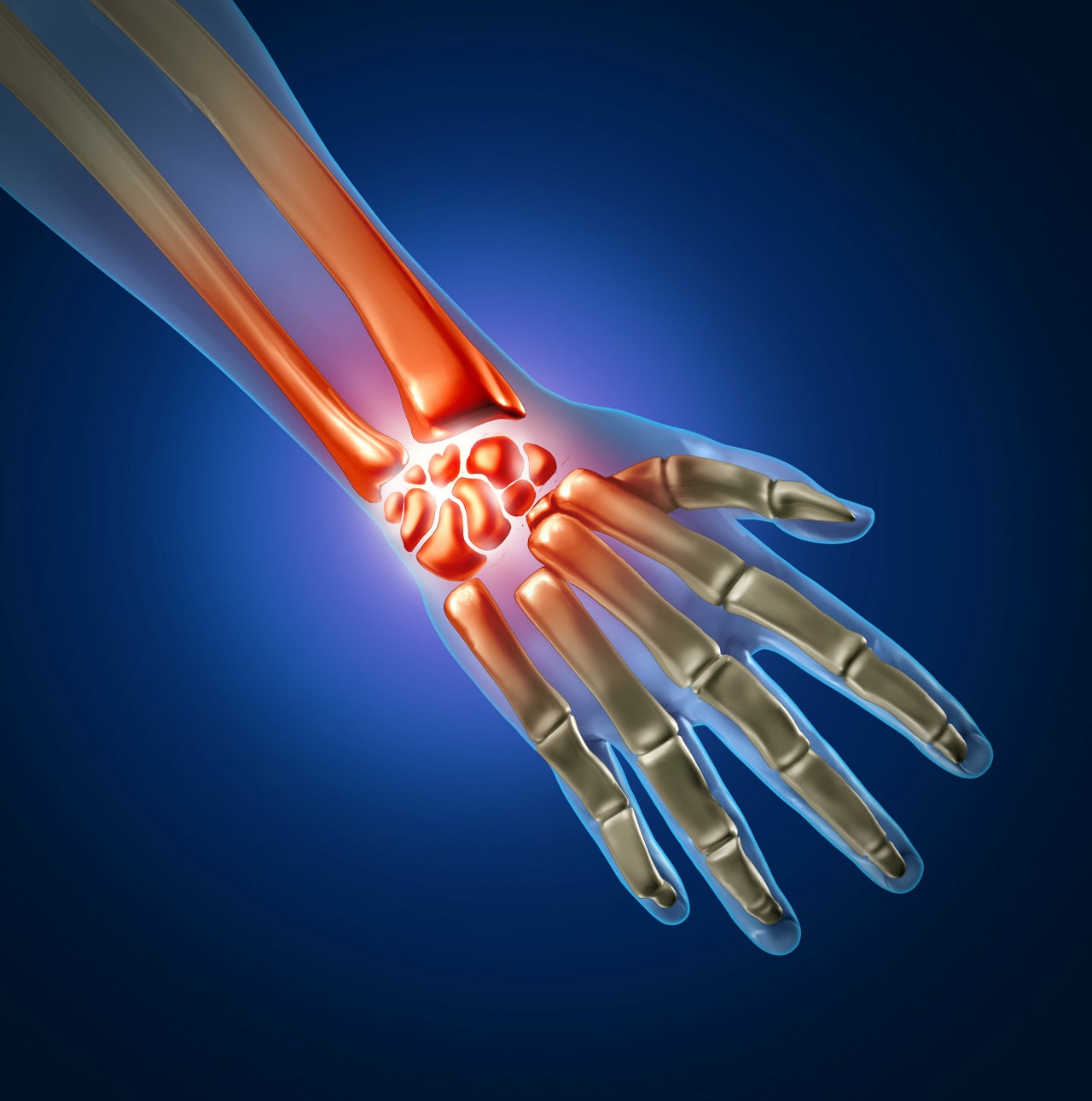
Anika Therapeutics has received 510(k) clearance from the Food and Drug Administration (FDA) for its WristMotion Total Arthroplasty System.
The modular joint restoration system is indicated for the replacement of painful wrist joint, resulting from rheumatoid arthritis, osteoarthritis, or post-traumatic arthritis.
It is designed to replace both radial and carpal portions of the joint.
WristMotion system brings together the company’s patented and proven fixation and dual curvature implant geometries with an instrumentation system that enables precise implant placement and joint tensioning.
Anika CEO and president Cheryl Blanchard said: “We are delighted to receive clearance for this innovative wrist device as it is another step forward in our mission to provide motion-preserving technologies.
“This is an underserved market and our solution is in response to demand from both orthopaedic surgeons and patients for solutions that avoid fusion and preserve as much natural motion and anatomy as possible.”
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe 510(k) clearance for the WristMotion system will lead to a one-time $5m earnout payment as part of the company’s merger agreement with Arthrosurface.
This earnout payment will be paid in the fourth quarter of this year.
In a separate development, orthopaedic device manufacturer Tyber Medical secured FDA 510(k) clearance for its new line of foot and ankle plating systems.
The clearance covers more than 42 different indication-specific anatomical plating families, which is developed using a combination of CT scans, cadaveric labs, and consultation with surgical thought leaders.
The plating systems are indicated for the treatment of a range of deformity, trauma, and degenerative conditions to the forefoot, midfoot, rearfoot, and ankle.
Tyber Medical plans to launch the first phase of plates in the first half of next year and expects to obtain CE Mark in Europe next year.






