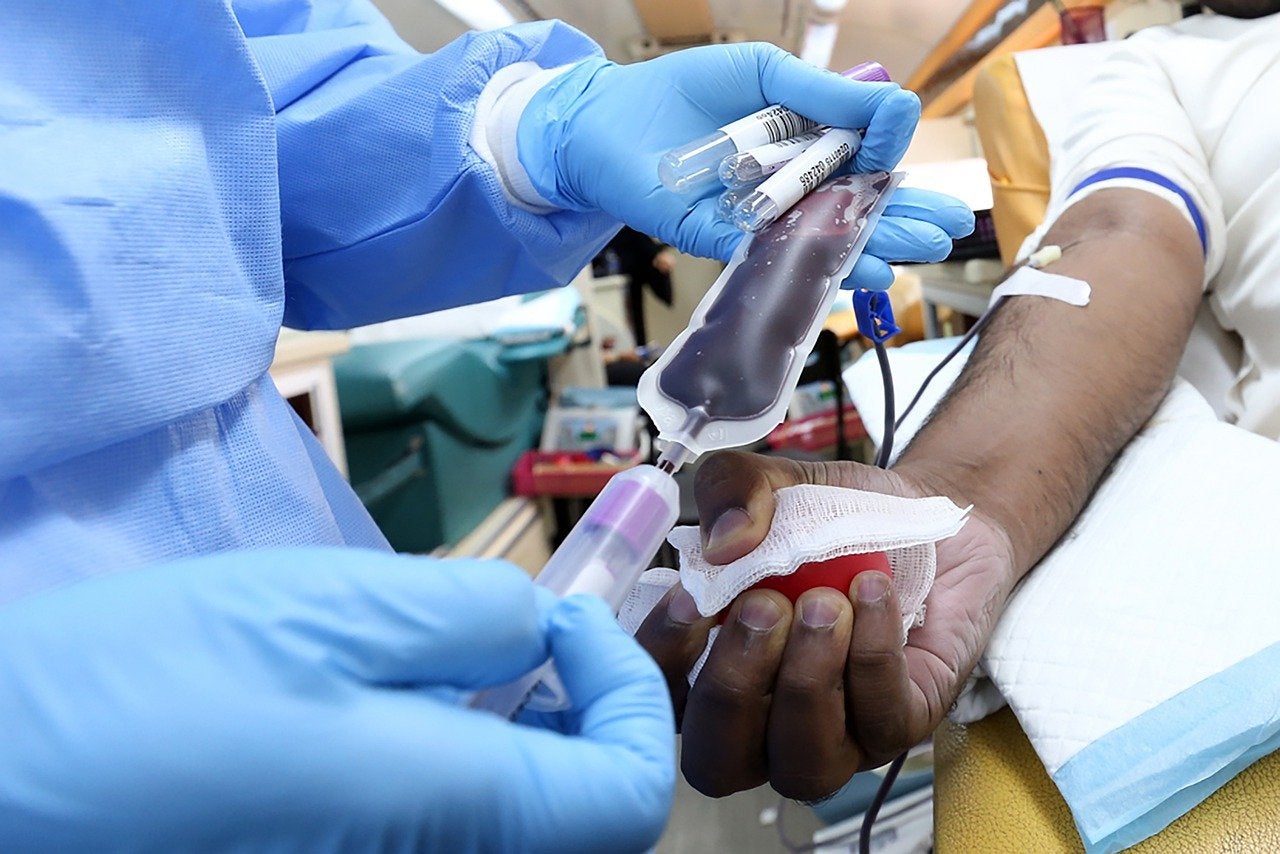
The US Food and Drug Administration (FDA) has granted approval for Cerus’ INTERCEPT Blood System for Cryoprecipitation.
The system, which secured breakthrough device designation, can be used to produce Pathogen Reduced Cryoprecipitated Fibrinogen Complex for treating and controlling bleeding, including massive hemorrhage, linked to fibrinogen deficiency.
Pathogen Reduced Cryoprecipitated Fibrinogen Complex stays transfusion-ready at room temperature for up to five days after thawing.
Cerus president and CEO William Greenman said: “FDA approval of the INTERCEPT Blood System for Cryoprecipitation is an important step forward in our mission to establish pathogen reduction as the standard of care for transfused blood components globally.
“We plan to begin selling Pathogen Reduced Cryoprecipitated Fibrinogen Complex in California, Texas, Louisiana, and Wisconsin in 2021 with expansion to national distribution in 2022, following anticipated approval of manufacturing site Biologics License Applications.”
Pathogen Reduced Cryoprecipitated Fibrinogen Complex is used in various other applications.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataIt is indicated for control of bleeding when recombinant and / or specific virally inactivated preparations of factor XIII or von Willebrand factor are not available.
In addition, it can be used for second-line therapy for von Willebrand disease and for control of uremic bleeding when other therapies fail.
Another derivative product from its production called Pathogen Reduced Plasma, Cryoprecipitate Reduced, also received approval from the FDA for transfusion or therapeutic plasma exchange in patients with thrombotic thrombocytopenic purpura (TTP).
Available across the world, the system for platelets and plasma is the only pathogen reduction system with both CE mark and FDA approval.



