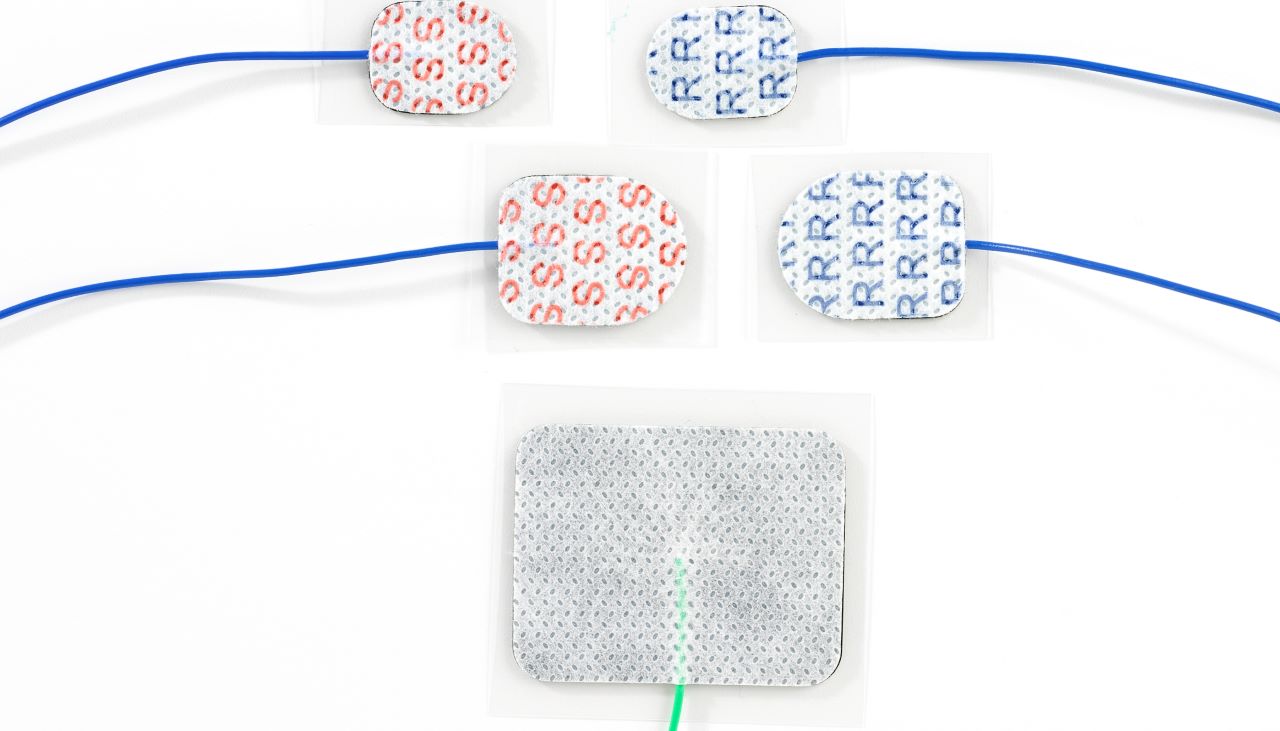
The US Food and Drug Administration (FDA) has granted clearance for medical device manufacturing company Rhythmlink International’s product line of peel and stick-on electrodes for Intraoperative Neurophysiological Monitoring (IONM) and Electroencephalography (EEG).
Sticky Pad Surface Electrodes are now FDA-Cleared MR Conditional for 1.5 and 3 Tesla MRI environments.
The clearance helped IONM professionals to use the three varieties of Sticky Pad electrodes, each designed for stimulating, grounding, or recording to obtain superior results.
Each recording pad is available pre-gelled in formulated hydrogel and records high-quality signals. The stimulating pad can reduce stimulation risks or artifacts.
To be available in three styles, the MR Conditional Sticky Pad electrodes are single-patient use and fully disposable. In addition, they can remain applied to the patient during the CT or MRI.
Rhythmlink sales and marketing vice-president Leah Hanson said: “In addition to being a lighter weight electrode with more surface area, the MR Conditional Sticky Pad Electrode can offer added patient comfort and protection to delicate skin areas by eliminating the need to remove and reapply the electrodes each time a CT or MRI is required.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“Because repeated removal and application can put the patient at a higher risk of skin breakdown and injury, it is precisely for these patients that it was crucial to make an electrode that can safely enter an imaging environment.”
In a separate development, the FDA has approved EndoClot Plus’ innovative product that helps gastroenterologists in stopping bleeding rapidly and reliably.
EndoClot Polysaccharide Hemostatic System (EndoClot PHS) is a single-use device with a starch-based powder hemostat applied to bleeding site using a flexible endoscope.
The patented Absorbable Modified Polymer (AMP) powder can rapidly concentrate the blood to expedite the normal physiological clotting process.
It is anticipated to be commercially available in the US by May.