Indian medtech company Meril’s MyVal-1 transcatheter heart valve (THV) has demonstrated a high procedural success rate in a one-month clinical trial.
The THV was trialled in 100 patients at intermediate or high risk for surgical aortic valve replacement (SAVR), and was seen to be highly safe and effective at a 30-day post-procedure follow up. Survival rate among patients was high, with a low incidence of stroke and a low rate of new permanent pacemaker implantation 30 days after the procedure.
Patients’ quality of life a month after implantation was also seen to be improved when measured by a six-minute walk test and the Kansas City Cardiomyopathy Questionnaire. The patients’ New York Heart Association (NYHA) classification score, which assesses the extent of heart failure, was also significantly improved.
Meril claims the success of the device lies in its precise orthotropic valve positioning for transcatheter aortic valve replacement (TAVR).
Meril vice president of corporate strategy Sanjeev Bhatt said: “The currently available TAVR systems have established safety and effectiveness in eligible patients. However, accuracy in valve sizing and precision placement as well as accuracy in deployment, with conduction system abnormalities leading to new permanent pacemaker implantation, paravalvular regurgitation and vascular complications remain challenging in certain settings.
“Myval THV is a next-generation TAVR system which is intuitive, minimising physicians’ learning curve and with promising clinical data that address most of these unmet needs.”
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
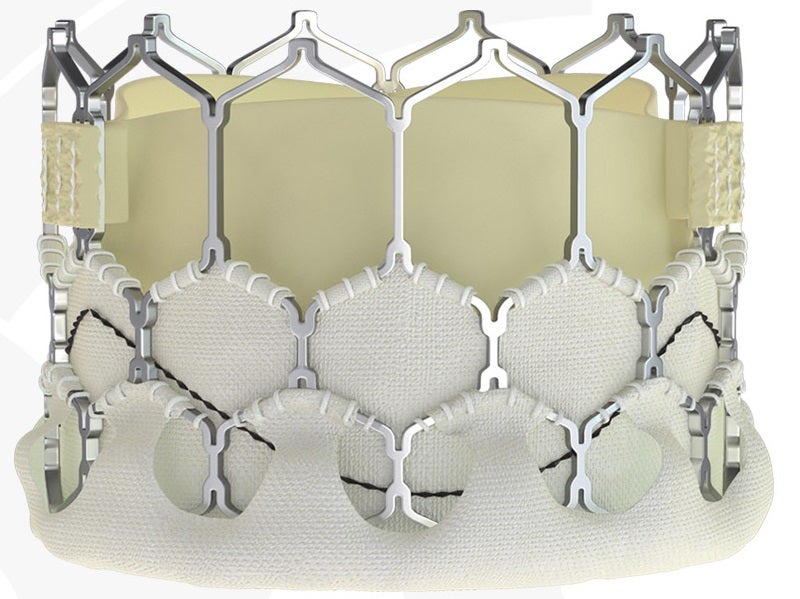
By GlobalDataThe MyVal-1 THV system is a balloon expandable transcatheter heart valve made of a nickel-cobalt alloy frame. Its hybrid honeycomb design with open cells toward the aortic end ensures un-jailing of the coronary ostia, while closed cells towards the ventricular end allow for high radial strength.
The device is equipped with an eternal PET sealing cuff for lower profile and puncture resistance and an external PET buffing to minimise paravalvular leaks.
The study results were presented at the PCR London Valves conference on 18 November by Eternal Heart Care Centre and Research Institute TAVR director Dr Ravinder Singh Rao.
“The direct crimping of the Myval THV balloon has made my TAVR procedures simple and intuitive,” said Rao. “Availability of intermediate sizes is a huge step in minimising patient prosthetic mismatch. Now we can size the prosthetic valve to the patient and not the other way around.
“Moreover, the ability to deliver all diameters from 20 – 29mm via a low profile 14Fr expandable introducer sheath has a profound impact in lowering vascular complications. I am enthusiastic about the excellent clinical and haemodynamic outcomes we have achieved at 30 days in the cohort of patients enrolled.”