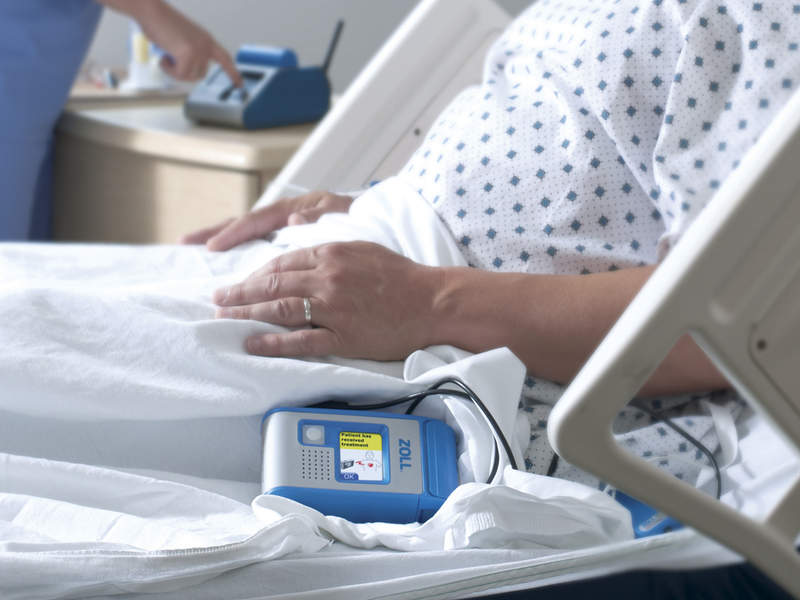

The US Food and Drug Administration (FDA) has granted premarket approval (PMA) to Zoll Medical's new Hospital Wearable Defibrillator (HWD).
The approval allows the firm to market and distribute the defibrillator in the US. The device was developed to protect patients at risk of ventricular tachycardia (VT) or ventricular fibrillation (VF) and it features automatic detection and immediate treatment, enabling continuous protection day or night.
Zoll Medical's chief executive officer (CEO) Jonathan Rennert said: “The HWD offers hospital care teams a new option for managing patients at risk of VT/VF by providing continuous protection even outside of very expensive, high-acuity care areas.
“It complements the tool kit the care team has in place for responding to cardiac arrest.”
The device is reported to have an ability to provide rapid defibrillation to hospitalised patients at risk for malignant ventricular arrhythmias. Upon detection of a life-threatening rhythm during VT or VF, HWD alerts the patient before delivering a treatment shock.
See Also:
While it is designed to provide the treatment shock within 60 seconds, a conscious patient can also choose to cause delay. The device utilises a detection algorithm and defibrillation waveform similar to that of the LifeVest wearable defibrillator, which reportedly demonstrated a 98% first shock success rate and 92% event survival rate.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataAn Asahi Kasei Group subsidiary, Zoll Medical develops and markets devices and software solutions for defibrillation, circulation, and cardiopulmonary resuscitation (CPR) feedback, as well as data and therapeutic temperature management and ventilation.
Image: The Zoll Hospital Wearable Defibrillator approved by FDA. Photo: courtesy of Business Wire/Zoll Medical Corporation.




