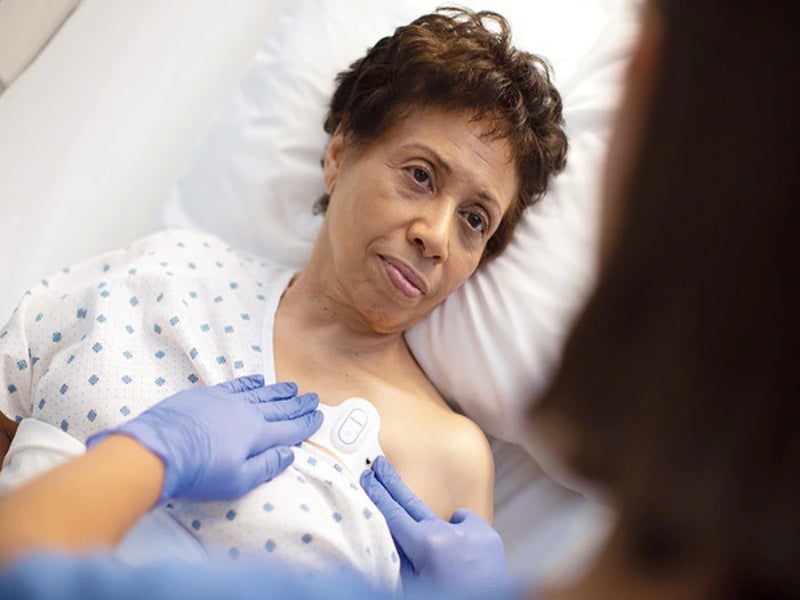
Royal Philips has received 510(k) clearance from the US Food and Drug Administration (FDA) for its wearable biosensor, Philips Biosensor BX100, to help monitor Covid-19 patients in hospitals.
Designed to address a new approach to vital signs measurements, the lightweight, disposable biosensor is a five-day single-use wearable patch.
It can be discreetly attached to the chest to collect, store, measure and transmit respiratory rate and heart rate every minute along with other contextual parameters such as posture, activity level and ambulation.
Philips Biosensor BX100 was designed for use by healthcare professionals on patients 18-years-old and older, the company noted.
The device will advance clinical surveillance in the Philips patient deterioration detection solution that also incorporates IntelliVue GuardianSoftware for early warning scoring and EarlyVue VS30 advanced patient monitors.
It will enable clinicians to help improve patient care in lower acuity care areas by detecting risk and making early interventions possible.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe company noted that the OLVG Hospital in the Netherlands is currently using the solution to help manage the triage and clinical surveillance of confirmed and suspected Covid-19 patients.
Philips Monitoring and Analytics general manager Peter Ziese said: “During this unprecedented time of Covid-19, the Philips Biosensor BX100 helps provide rapid deployment for clinical surveillance to help decrease the risk of exposure of healthcare workers while acquiring frequent patient vitals and easing the demand for personal protective equipment (PPE).
“The biosensor is an integral component in our patient deterioration detection solution, which helps in the identification of the subtle signs of deterioration in a patient’s condition at the point of care, hours before a potential adverse event would occur.”
The Philips wearable biosensor is part of the company’s portfolio of solutions to address patient deterioration, supporting general care around the world with smart clinical intelligence.