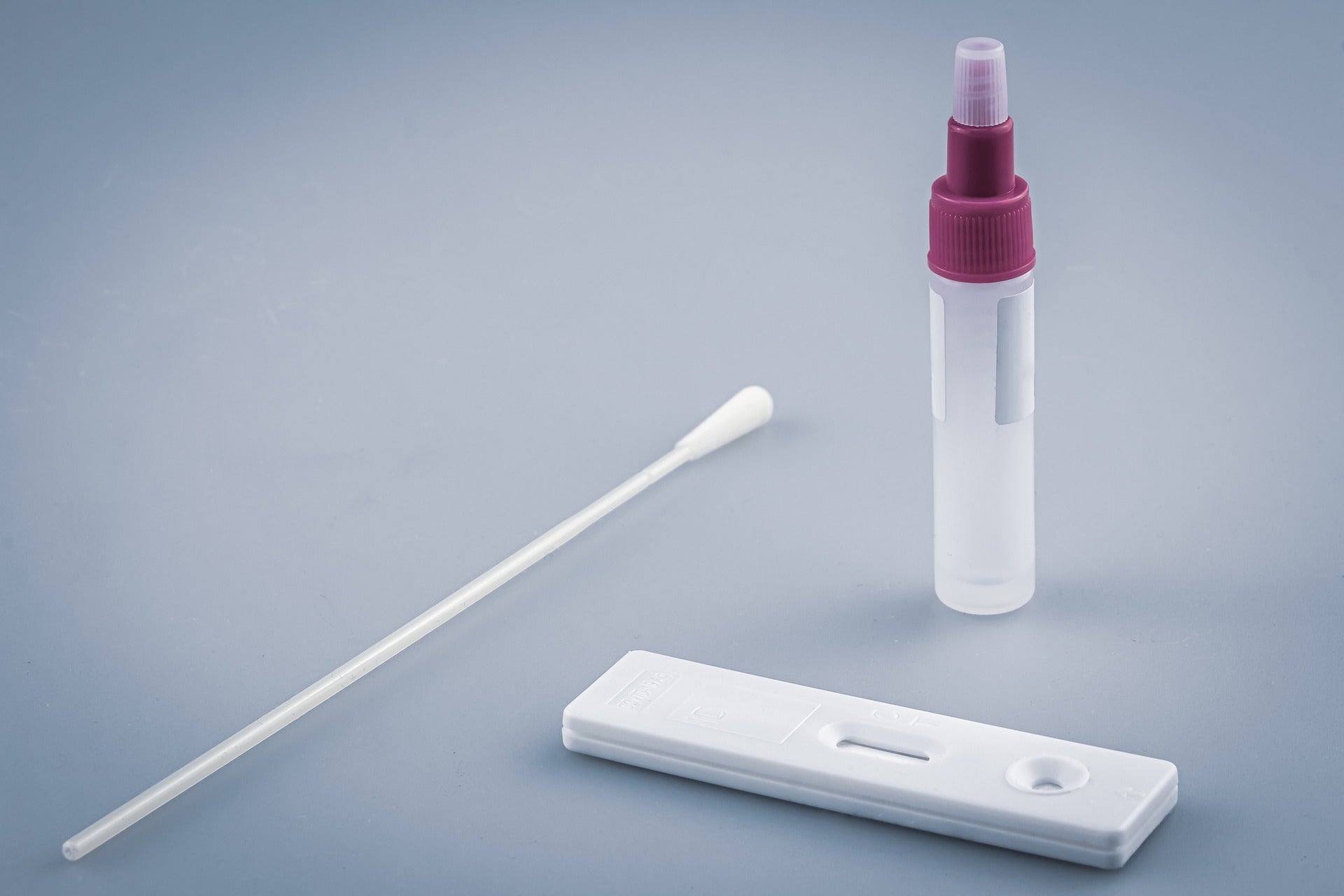
The Covid-19 Technology Access Pool (C-TAP) of the World Health Organization (WHO) and the Medicines Patent Pool (MPP) have finalised a worldwide, non-exclusive licencing agreement with the Spanish National Research Council (CSIC) for its Covid-19 serological antibody test.
The test can detect the presence of anti-Covid-19 antibodies generated in response to either a SARS-CoV-2 infection or vaccination.
The non-exclusive licensing agreement is the first-ever test licence to be signed by the MPP.
The WHO noted that the latest move will aid in the quick production and marketing of the CSIC Covid-19 serological test globally.
All associated patents, as well as the biological material required for the test’s production, are included in the deal.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe CSIC will extend complete expertise and training to MPP and/or other potential licensees.
For low- and middle-income nations, the licence will be offered on a royalty-free basis and remain valid until the final patent expires.
So far, the technology has yielded four separate tests. One among them can differentiate the immune response of Covid-19 infected people from those who have received the vaccine.
The easy-to-use tests could be used in various settings, such as rural regions in low- and middle-income nations, with simple lab infrastructure.
The test results can be read manually with the naked eye, however, an ELISA reader is advised for improved precision.
WHO director-general Dr Tedros Adhanom Ghebreyesus said: “I highly commend CSIC, a public research institute, for its commitment to solidarity and for offering worldwide access to their technology and know-how.
“This is the kind of open and transparent licence we need to move the needle on access during and after the pandemic.
“I urge developers of Covid-19 vaccines, treatments and diagnostics to follow this example and turn the tide on the pandemic and on the devastating global inequity this pandemic has spotlighted.”
In October, Fulgent Genetics unveiled a new lab-developed at-home Covid-19 neutralising antibody test.



