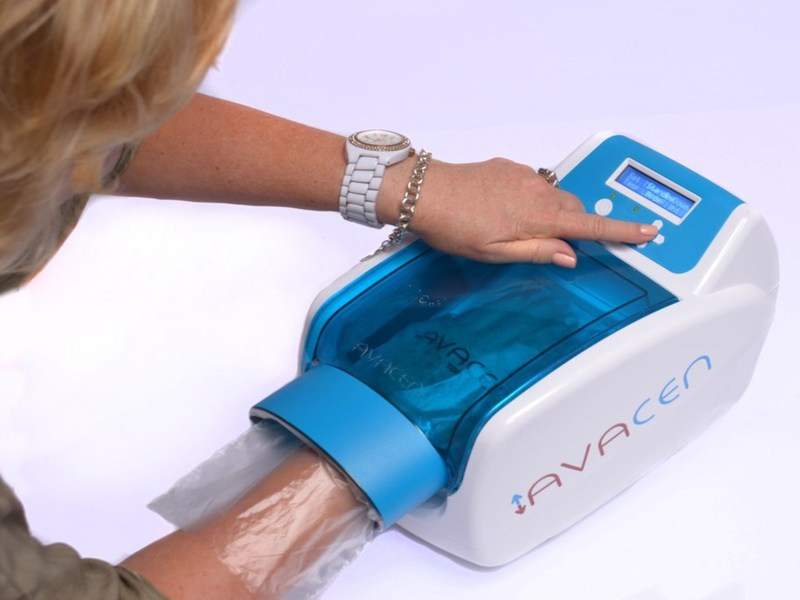

US-based pain relief solutions provider Avacen Medical has secured medical device licence approval from Health Canada for its new pain treatment device, Avacen 100.
The new CE-marked device employs the firm’s new treatment method in which heat is non-invasively and safely induced into the circulatory system.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The infusion of heat leads to muscular relaxation and increase of microcirculation in the body.
According to Avacen Medical chief executive officer Thomas Muehlbauer, the device allows whole body treatment with a single point of contact.
Muehlbauer said: “It is the ideal drug-free and safe alternative for relief of muscle and joint pain.
“The most exciting aspect of this approval is that the Canadian market has shown high demand and acceptance for innovative new alternatives to pharmaceuticals for treating pain.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe firm intends to further seek Health Canada’s approval to extend the use of Avacen 100 for the treatment of fibromyalgia, which is a chronic widespread pain.
The device was found to significantly minimise this kind of pain during a clinical study conducted in 22 subjects suffering from fibromyalgia.
It was found to relieve pain associated with pinched nerves, stress, panic disorder and generalised anxiety, and is also expected to provide relief from joint pain caused by CRPS, Reynaud’s and Lyme disease.
In addition to Health Canada, the US Food and Drug Administration (FDA), India FDA and European Union (EU) approved the device for temporary relief of minor muscle and joint pain, stiffness, muscle spasms, joint pain related to arthritis, as well as minor strains and sprains. They also approved the device for the temporary increase of local circulation and muscular relaxation.
EU approval also includes temporary relief from widespread pain and temporary increase of microcirculation.
Image: Avacen 100. Photo: courtesy of PRNewsfoto/Avacen Medical.





