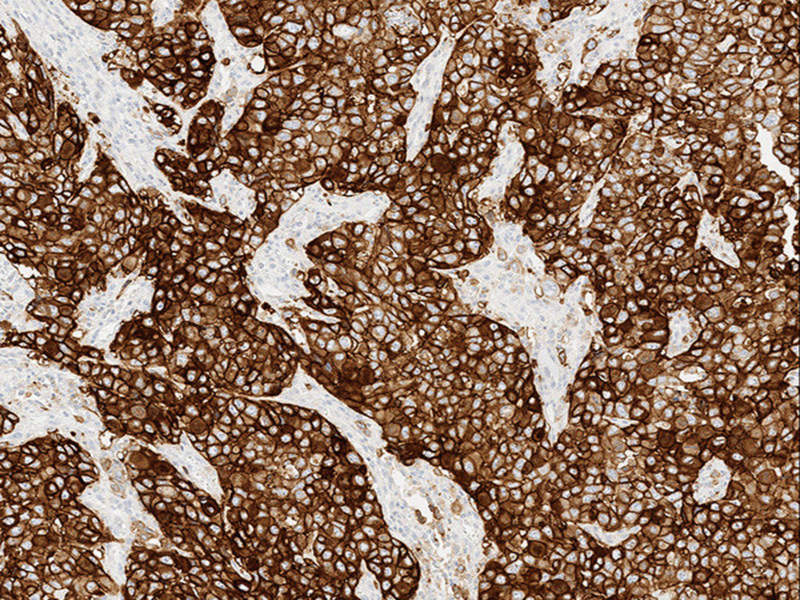

The US Food and Drug Administration (FDA) has granted approval to Roche's new biomarker test VENTANA PD-L1 (SP263) as a complementary diagnostic to identify programmed death ligand (PD-L1) status in urothelial carcinoma patients.
The approval is valid for locally advanced or metastatic urothelial carcinoma (mUC) patients who are potential candidates to receive treatment with Imfinzi (durvalumab), AstraZeneca's anti-PD-L1 immunotherapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The test uses tumour and immune cell staining, as well as scoring in the tumour microenvironment to examine the status of PD-L1.
Roche tissue diagnostics head Ann Costello said: "Urothelial carcinoma is an area of significant unmet medical need.
"We are very pleased the VENTANA PD-L1 (SP263) has received FDA approval as it will serve as a powerful tool to help inform physicians about appropriate treatment options for their patients."
The diagnostics firm is working towards obtaining regulatory approval for its PD-L1 (SP263) assay in other cancer indications in the country, as well as international markets.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataRoche Diagnostics' UK and Ireland division has introduced a new self-monitoring device called CoaguChek INRange metre for patients receiving warfarin anticoagulant therapy.
Through an app on their phone, patients can reduce the number of clinic visits by sending their results using a wireless connectivity.
A survey carried out by Atrial Fibrillation Association (AFA) found that 86% of patients on warfarin prefer self-monitoring to avoid clinic visits.
CoaguChek INRange metre enables patients to set reminders and add comments to specific results.
Image: VENTANA PD-L1 (SP263) Assay staining in urothelial carcinoma. Photo: courtesy of PRNewswire/Roche.





