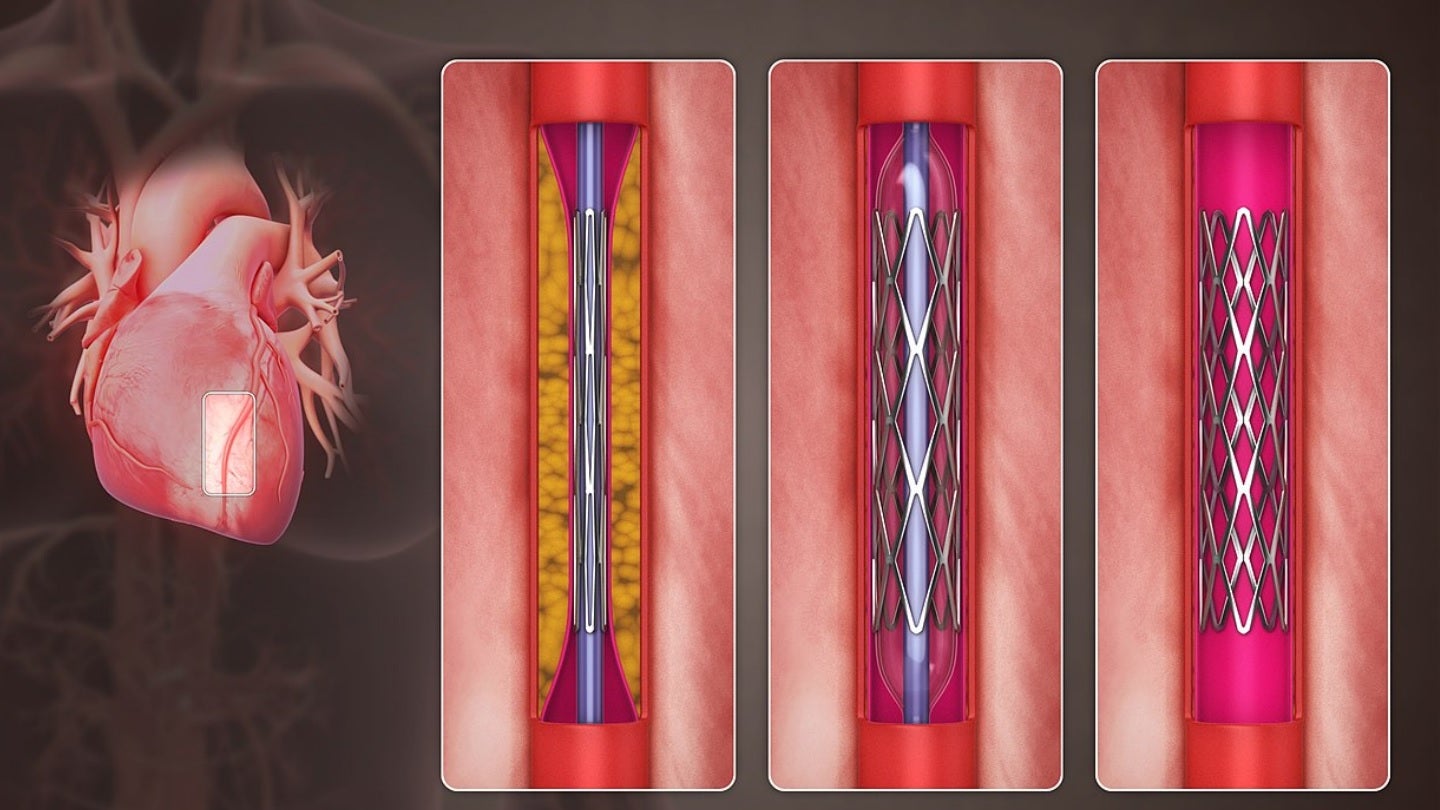
Pulse Medical Technology (Pulse Medical) has received breakthrough device designation (BDD) from the US Food and Drug Administration (FDA) for its fourth-generation μFR system.
With a broad range of indications, the μFR is an angio-based physiological assessment tool that is free from a pressure wire or hyperemic agents.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company developed the algorithm of μFR, which offers a quick computation methodology for fractional flow reserve (FFR) using multiple imaging data.
μFR is suitable for use across the different stages of the percutaneous coronary intervention (PCI) procedure.
It can be used to perform accurate physiology evaluation at Pre-PCI, provide strategy optimisation during the operation, outcome and microcirculatory function assessment at post-PCI.
Pulse Medical president Bing LIU said: “We are delighted that μFR has been designated an FDA breakthrough device.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Cardiovascular diseases are the leading cause of death globally; an estimated 17.9 million people died from that in 2019. μFR as a physiological assessment tool could provide more insights to physicians and help more patients have an effective and precise treatment.”
According to a one-year follow-up of the FAVOR III China clinical trial, μFR demonstrated a significant 35% reduction in MACE risk.
The group of patients guided by μFR achieved improved prognostic outcomes.
Established in 2015, Pulse Medical is involved in the development of advanced technology for accurate diagnosis and optimal treatment of pan-vascular disease patients.



