RevBio has secured approval from the US Food and Drug Administration to start a first-in-human clinical trial for its regenerative bone adhesive for cranial flap fixation.
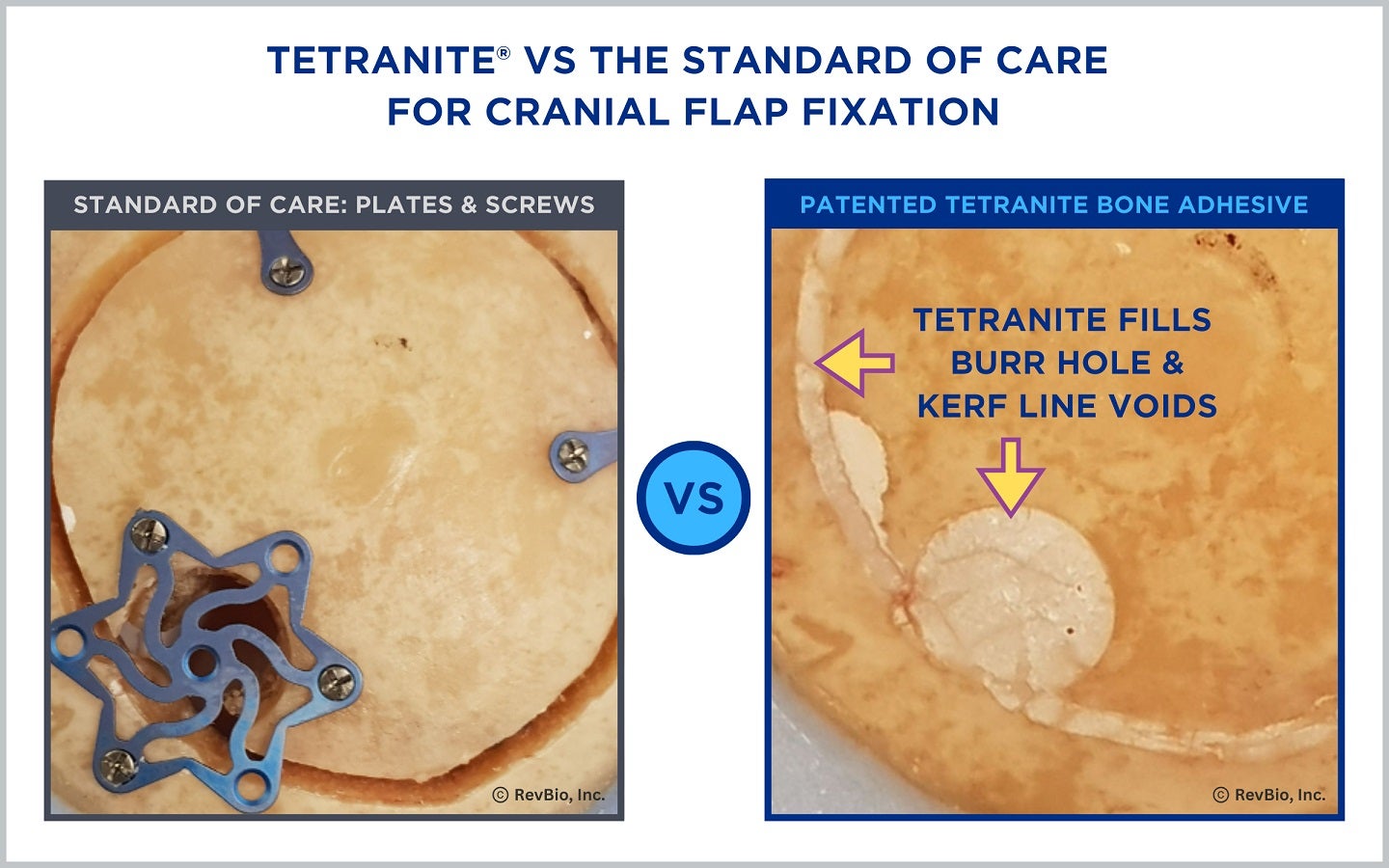
The 20-patient pilot study aims to assess the probable benefit and safety of the bone adhesive biomaterial, Tetranite, to replace the use of metal plates and screws.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The biomaterial is designed to stabilise and fixate cranial flaps immediately after craniotomy procedures related to brain surgery.
These procedures involve temporary removal of a part of the skull, and the usual way to put it back involves using plates and screws.
However, this method can leave a gap between the bone and the skull, leaving the possibility for cerebrospinal fluid (CSF) to leak and cause infections. These infections can be serious, causing problems such as meningitis, subdural empyema, or abscesses in the brain.
RevBio’s Tetranite could serve as an alternative method for cranial flap fixation that eliminates the use of hardware fixation products while providing sealant to resist CSF leaks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIts use is also expected to improve bone healing and improve the conformal contour of the skull.
RevBio CEO and founder Brian Hess said: “We envision that the use of Tetranite will become the standard of care given all the benefits this biomaterial can provide in comparison to traditional plates and screw systems.”
The trial is planned to be carried out by Brigham and Women’s Hospital in Boston, Massachusetts Computational Neuroscience Outcomes Center director and practising neurosurgeon Timothy Smith; and Methodist Brain and Spine Institute cranial base surgery co-director and Semmes Murphey Clinic practising neurosurgeon L Madison Michael.
Smith said: “We are looking forward to evaluating the efficacy of the product that could provide immediate, rigid fixation that would be preferable to existing plates and screws, which are the current standard of care.
“We’re excited at the potential that this product could provide a biological adherence between the bone flap edges and the skull, as well as an architecture for osteogenesis over time and look forward to the results of the clinical trial.”





