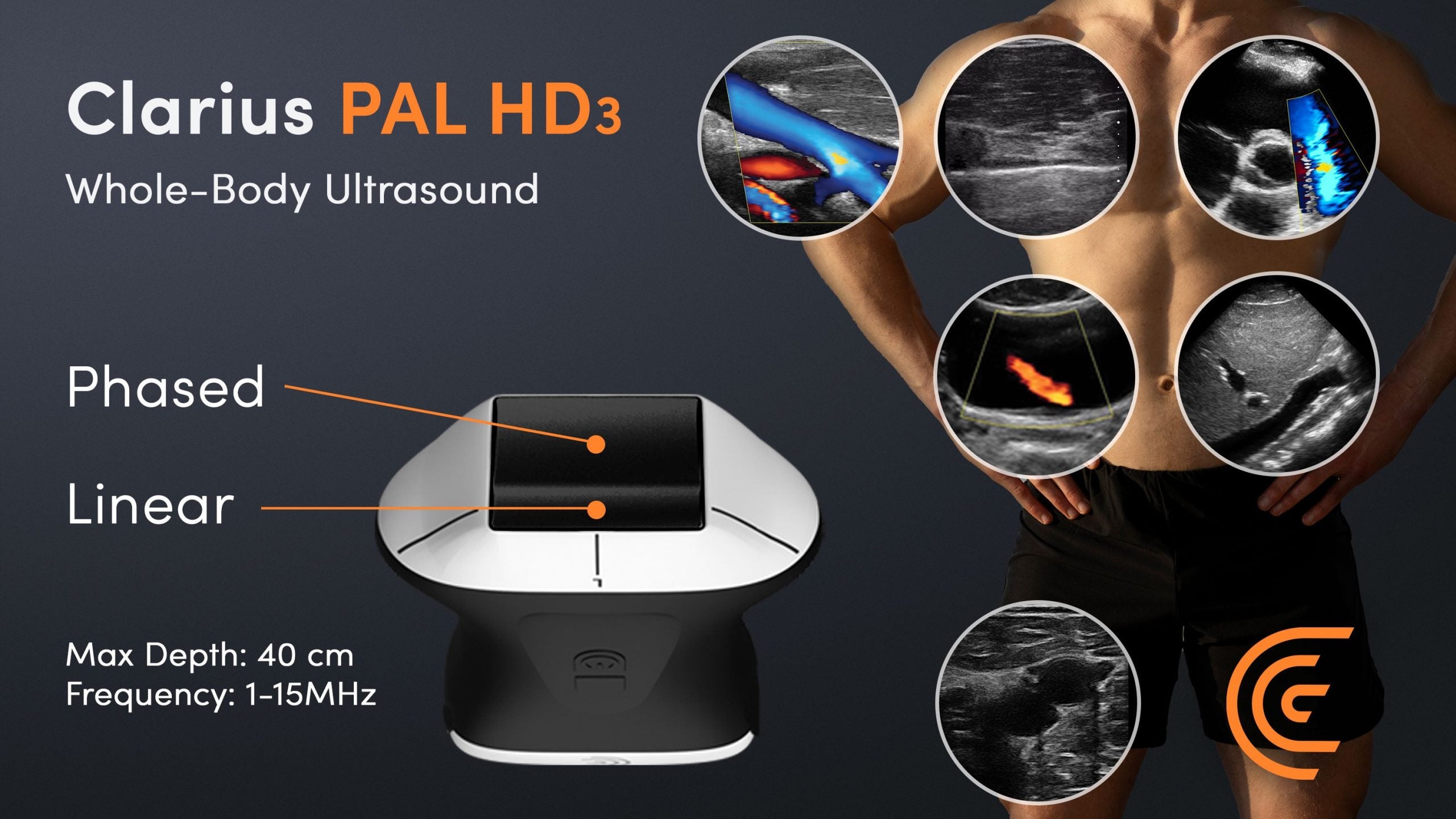
Canadian Clarius Mobile Health has received a CE mark for its wireless handheld whole-body ultrasound scanner, Clarius PAL HD3.
Concurrently the company has also launched the device in the European Union and the UK, as per a 17 January press release. The Claruis’ handheld device can provide high-resolution images up to 40 cm in depth from the skin surface.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The PAL HD3 device uses an eight-beamformer processing technology, a radio frequency (RF) technique that allows wireless signals to be targeted toward a specific endpoint. The beamforming technology is also used by the higher-end traditional counterparts.
In January, the company received 510(k) clearance from the US Food and Drug Administration (FDA) for its new Clarius Bladder AI software. The non-invasive tool can automatically measure bladder volume and can be used to monitor urinary retention and assess bladder emptying in patients with neurogenic bladder or urinary tract obstruction.
The market for ultrasound systems is forecast to grow from being worth approximately $4.7bn in 2023 to be worth over $5.4bn in 2030, as per GlobalData market analysis. Multiple companies have developed portable ultrasound systems.
In August 2023, ALPINION Medical Systems debuted its portable ultrasound diagnostic device, X-CUBE i9. The device has dual batteries and probe connectors that improve the speed of examinations. It can operate for 100 hours in standby mode and more than one hour in scan mode using the two batteries.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTo further increase the efficiency of ultrasound systems, companies are developing integrations with artificial intelligence (AI). In October 2023, GE HealthCare signed a contract worth $44m with the Biomedical Advanced Research and Development Authority (BARDA) to develop AI-enabled ultrasound technology. The integrated AI software is expected to improve interpretations of the exams, particularly in trauma cases.
Ultrasound technology is also being developed for therapeutic purposes. Vibrato Medical is developing a wearable non-invasive ultrasound device to treat chronic limb-threatening ischemia (CLTI). The device trial met its primary endpoint by demonstrating an improvement in toe perfusion and an increase in tissue oxygenation by 17%.
Cardiawave is developing a non-invasive ultrasound therapy as a treatment for calcific aortic stenosis. In November 2023, the French company initiated a pivotal trial for the device across multiple European sites.





