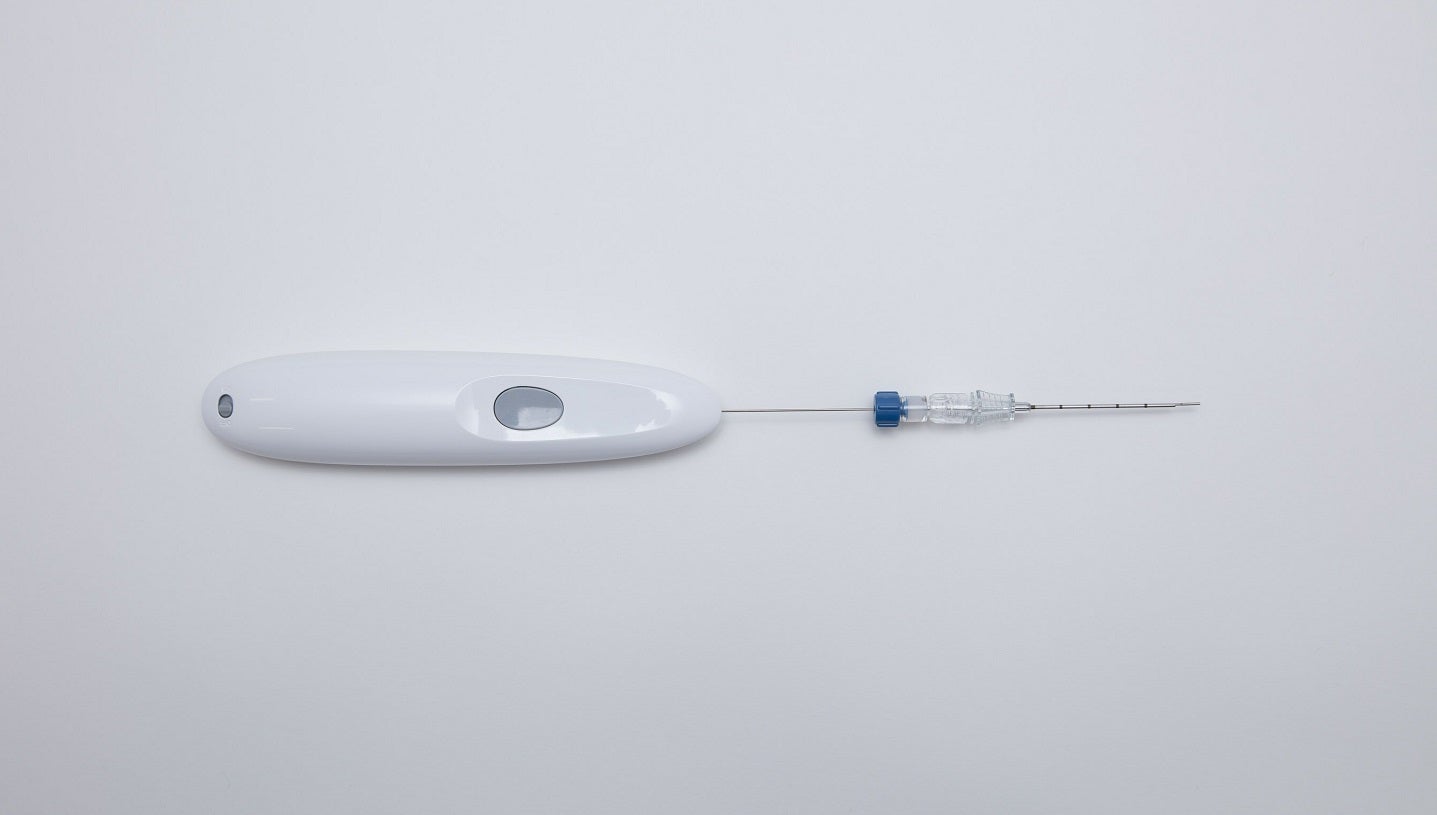
Medical devices company Single Pass has entered an exclusive distribution agreement with Mermaid Medical Group to bring its advanced biopsy closure device to the US market.
The partnership aims to enhance patient care and improve procedural outcomes in biopsy closures.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
With its extensive network, Mermaid Medical Group will be the sole distributor of the Single Pass biopsy closure device in the US.
The company will also ensure widespread availability of the device to healthcare facilities in the country.
Single Pass CEO Bill Colone said: “Mermaid Medical Group’s reputation for excellence and their commitment to advancing patient care aligns perfectly with our mission. Together, we aim to revolutionise biopsy closure procedures and improve patient outcomes.”
The device will be more accessible to healthcare professionals by leveraging Mermaid Medical Group’s distribution capabilities to offer an advanced solution for biopsy closure procedures.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Single Pass biopsy closure device is designed to offer healthcare professionals a safe and efficient method for closing biopsy sites.
The user-friendly design and advanced technology of the device are expected to reduce the risk of complications and promote faster patient recovery.
Mermaid Medical Group general manager Marcus Nielsen said: “We are excited to collaborate with Single Pass and introduce their groundbreaking biopsy closure device to the US market.
“This partnership reflects our dedication to providing healthcare professionals with innovative solutions that enhance patient care. We look forward to working closely with Single Pass to make this device widely accessible to medical facilities across the country.”
With a focus on advanced solutions development for biopsy closure procedures, the company aims to revolutionise the interventional medicine field.





