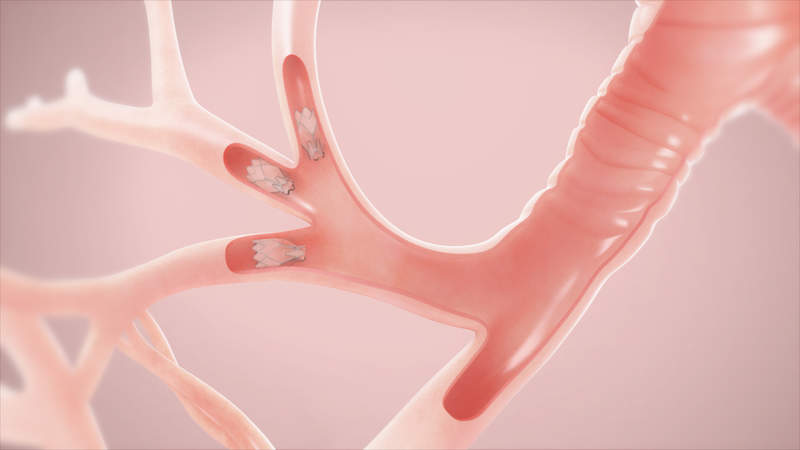
Pulmonx, an interventional pulmonology treatments provider, has secured the US Food and Drug Administration (FDA) approval for its Zephyr Endobronchial Valve System to treat severe emphysema, a type of chronic obstructive pulmonary disease (COPD).
Zephyr has been designed as a minimally invasive bronchial valve that can be implanted in the airways during a bronchoscopic procedure to occlude a diseased part of the lungs and minimise hyperinflation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
During inhalation, the valves close and prevent entry of air into the damaged part and during exhalation, the valves open to let out trapped air.
This mechanism is said to aid the healthier parts of the lungs to expand, thereby lifting pressure off the diaphragm and in turn reducing shortness of breath.
FDA Anesthesiology, General Hospital, Respiratory, Infection Control and Dental Devices division acting director Tina Kiang said: “Treatment options are limited for people with emphysema who have severe symptoms that have not improved from taking medicines.
“This novel device is a less invasive treatment that expands the options available to patients.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe regulatory decision comes after a review of positive clinical data from the LIBERATE Study and two other multi-centre randomised control trials.
Data from LIBERATE showed more patients treated with Zephyr Valves and medical management could breathe easier, were more active, experienced lesser shortness of breath and had a significant improvement in the quality of life when compared to those who received only medical management.
Some of the adverse events observed during the study were death, air leak or pneumothorax, worsening of emphysema, pneumonia, coughing up blood, chest pain and shortness of breath.





