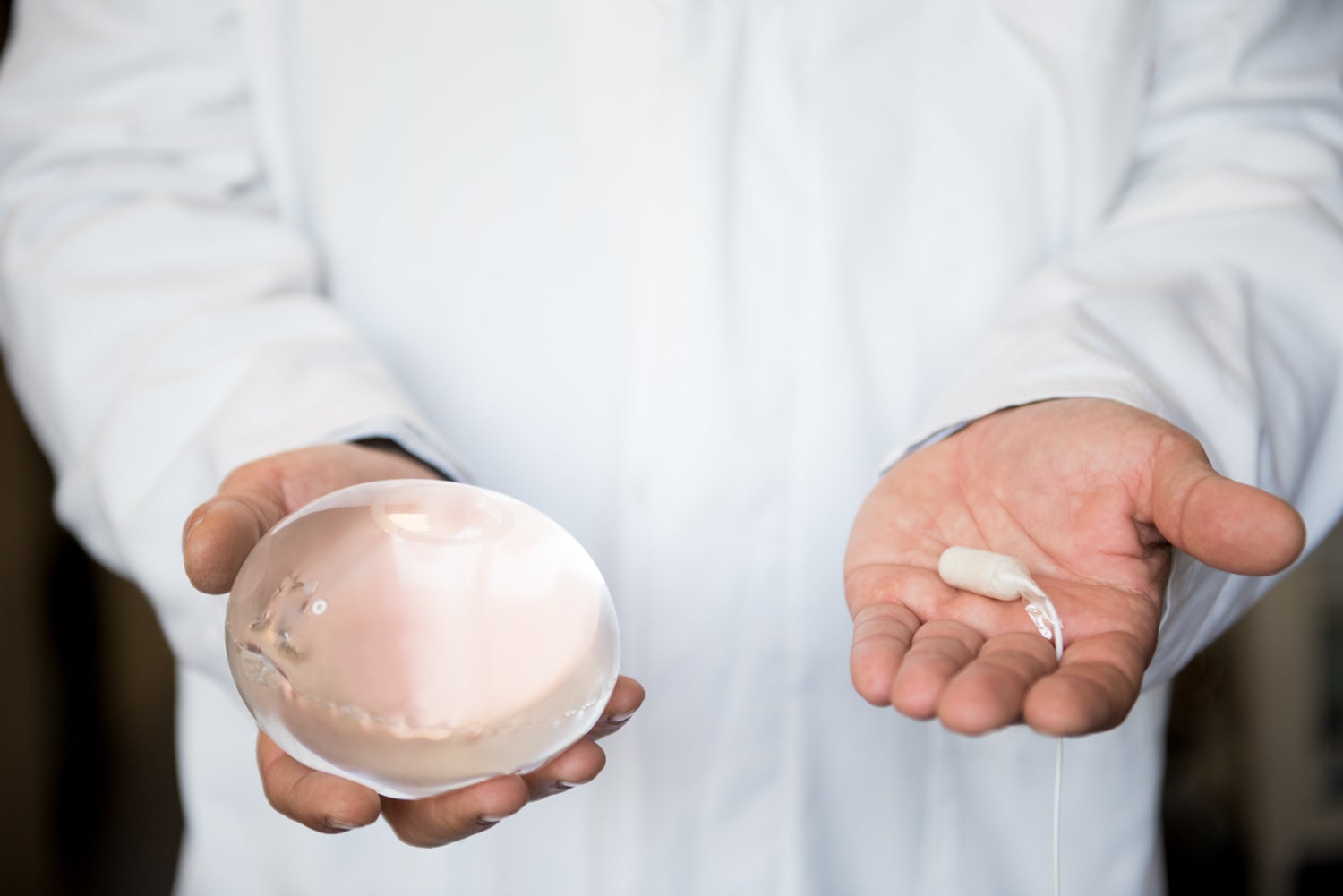
Allurion’s intragastric balloon has demonstrated yet more positive efficacy and safety results in a study on patients living obesity, though the company continues to struggle with weakening financial performance.
The single-centre retrospective study involved 167 patients in Ecuador with an average body mass index (BMI) of 31.3. Results, published in Clinical Obesity, showed that after four months of therapy with the balloon – which works by making patients feel full – participants achieved an average weight reduction of 15.7%.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Patients continued to lose weight after the balloon had passed through the body, with an average weight loss of around 17% at six months.
In terms of shedding more than 50% of excess body weight, 92 patients met this goal by month four. By month six, this number had risen to 104 patients.
However, the researchers of the paper noted that the study also showed the need for “further research regarding weight behaviour after [balloon] expulsion,” noting that the average weight loss after a year decreased to 14.7%.
Allurion’s gastric balloon has been available in Europe since it received CE marking in 2015. It is swallowed as a capsule and then filled with liquid. From there, it acts like a traditional gastric balloon that promotes weight loss by mimicking a full stomach. Unlike other approaches, Allurion’s balloon does not require surgery, endoscopy, or anaesthesia. After around four months, the balloon releases the liquid on its own and it passes out of the body naturally.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDespite solid efficacy and safety, Allurion has struggled to cope with the popularity of GLP-1 weight loss drugs such as Wegovy (semaglutide) and Mounjaro (tirzepatide). The former drug, manufactured by Novo Nordisk, generated $4.5bn in sales in 2023.
Just over a year after going public on the New York Stock Exchange (NYSE), the company received a delisting warning for falling short of share price compliance standards in August. Shares in the company are trading 84% lower compared to the same time last year. Allurion has a market cap of $45.6m.
Total revenue for Q4, 2023 was $8.2m for Allurion, compared to $19.2m for the same period in 2022. At the time it reported the news, the company’s CEO Shantanu Gaur admitted the quarter was impacted by “macroeconomic conditions and the surge of attention paid to GLP-1 drugs”.
Allurion has since highlighted the usefulness of its balloon in adolescents, a group for whom weight loss drugs are not yet approved. The company published data last month showing its device led to a 13.1% reduction in weight in teenagers living with obesity.
Allurion has also completed enrollment in a separate US pivotal trial called AUDACITY to support future US Food and Drug Administration (FDA) approval.





