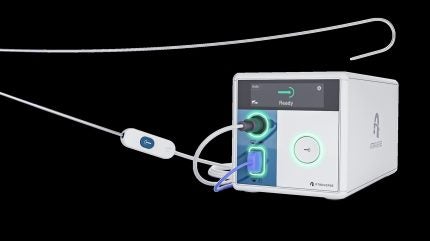
Atraverse Medical’s fully integrated HOTWIRE transseptal access system has obtained the US Food and Drug Administration’s (FDA) 510(k) clearance.
The system consists of the HOTWIRE RF generator, which is described as the first left-heart access system utilising impedance-guided technology that stops the delivery of energy once transseptal crossing is achieved.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This aims to reduce needless exposure to radiofrequency (RF) in the left atrium and allows clinicians to control energy activation within the sterile field.
The system also features the zero-exchange HOTWIRE RF guidewire, which is compatible with a wide range of sheaths.
It incorporates a tip design that is said to improve echocardiographic visualisation by 25%, along with a reinforced core wire and polymer jacket.
The HOTWIRE RF Guidewire, which received clearance from the FDA in May 2024, is said to have been used in nearly 2,000 clinical procedures.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSt Bernard’s Heart and Vascular Center’s cardiac electrophysiology director Dr Devi Nair, performed the initial clinical cases using the complete HOTWIRE system.
Dr Nair said: “Gaining safe and precise access to the left atrium remains one of the most critical steps in both electrophysiology and structural heart interventions.
“In my clinical experience, the HOTWIRE system delivers a level of control, accuracy, and procedural ease that meaningfully elevates the transseptal workflow. I look forward to sharing these results at the AF Symposium, for I believe this technology represents a true advancement in transseptal access.”
Atraverse Medical COO, co-inventor and co-founder Eric Sauter said: “The FDA clearance of the HOTWIRE system is a significant milestone for Atraverse — one made possible by the unwavering dedication, ingenuity, and technical excellence of our development team.
“After three years of focused innovation, our integrated end-to-end system and intentionally-engineered RF generator now provides a seamless solution to a long-standing clinical challenge — unlocking new opportunities for commercial success and meaningful impact for clinicians and patients.”





