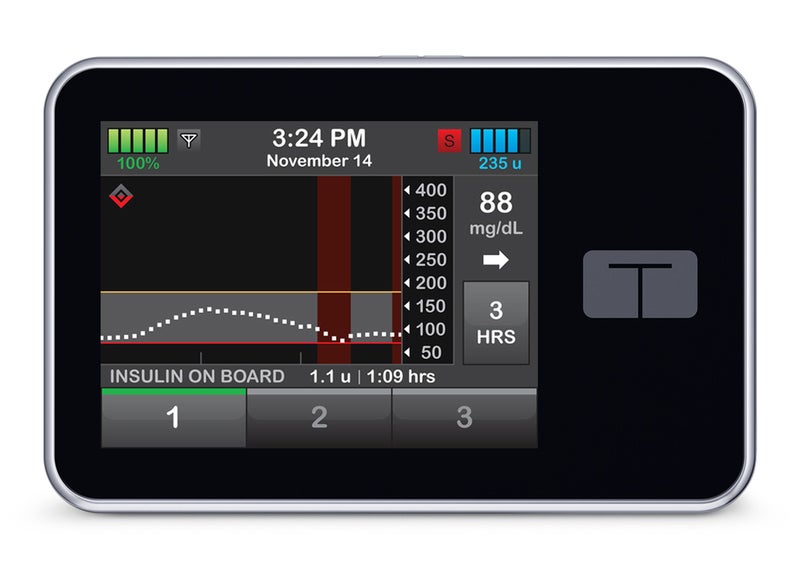
The US Food and Drug Administration (FDA) has granted marketing clearance for the Tandem Diabetes Care’s t:Slim X2 insulin pump, which can deliver insulin under the skin in children and adults with diabetes.
The diabetes technology company noted that the t:Slim X2 is the first under the new alternate controller enabled infusion pumps (ACE pumps) category.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It features an interoperable technology that enables use with various components of diabetes therapy systems, which may include an ACE insulin pump and other compatible medical devices such as automated insulin dosing (AID) systems and continuous glucose monitors (CGMs).
This capability facilitates the tailoring of diabetes management based on individual preferences.
The device delivers insulin at set or variable rates and can digitally connect with other diabetes management devices for automatic communication and to receive drug dosing commands.
FDA commissioner Scott Gottlieb said: “The marketing authorisation of the first ACE insulin pump intended for interoperable use has the potential to aid patients who seek more individualised diabetes therapy systems and opens the door for developers of future connected diabetes devices to get other safe and effective products to patients more efficiently.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe regulatory agency reviewed t:Slim X2 performance data that showed accurate and reliable dosing of insulin at the rates and volumes programmed by the user.
It also evaluated the capability of the pump to communicate with external devices with suitable reliability, cybersecurity and fail-safe modes.
The FDA is also establishing criteria called special controls that outline requirements for validating the accuracy, reliability, cybersecurity and clinical relevance of ACE infusion pumps.
In addition, the criteria provide information on the type of studies and data needed to demonstrate the acceptable performance of the pumps.
Diabetes is just one of the most prevalent chronic diseases that is seeing an increase in technological treatments for the condition. Some of the latest medical devices for diabetes include high-tech insulin pens, management apps and glucose monitors.
Additional reporting by Charlotte Edwards.





