The Australian Therapeutic Goods Administration (TGA) has approved Mainstay Medical’s ReActiv8 device, an active implantable medical device used to treat patients with chronic low back pain (CLBP).
The approval confirms enlistment of the device in the Australian Register of Therapeutic Goods (ARTG), which will enable its commercialisation in Australia.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
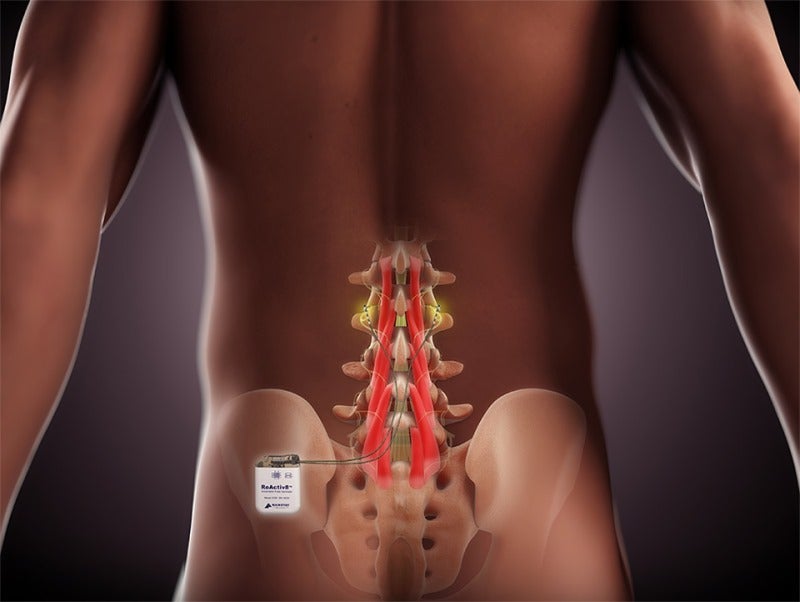
ReActiv8 is an implantable restorative neurostimulation system, which electrically stimulates the nerves meant for contracting muscles that stabilise the lumbar spine.
The system is designed to help patients regain control over muscles that provide lower back stability.
ReActiv8 targets patients who have no indications for spine surgery or spinal cord stimulation and have continuing pain despite medical treatments.
Dublin-based Mainstay Medical intends to add ReActiv8 to the Australian Prostheses List of reimbursed products.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe firm secured CE Mark for ReActiv8 in May 2016 based on positive results from the ReActiv8-A clinical trial, which indicated a lasting improvement in pain, disability and quality of life in people with disabling CLBP.
In August, the firm submitted the final module of its Pre-Market Approval (PMA) application to the US Food and Drug Administration (FDA).
Mainstay Medical CEO Jason Hannon said: “Australian physicians who have been part of our clinical studies to date are among the most experienced globally in selecting and treating patients with ReActiv8 therapy.
“The clinical data in support of ReActiv8 continues to build and was instrumental in demonstrating to TGA that ReActiv8 is a valuable therapy that should be available to Australian patients.
“This approval was received ahead of our expected timeline, and we will now move to the next step in the process, which is applying for inclusion of Reactiv8 on the Prostheses List. We plan to launch ReActiv8 commercially after securing a place on the Prostheses List.”





