HeartMate 3™ is an advanced left ventricular assist device (LVAD) or heart pump based on a technology known as Full MagLev™ (fully magnetically-levitated) Flow.
It is a mechanical circulatory support device approved for advanced heart failure patients who require short or long-term mechanical cardiovascular support, such as bridge to transplant or destination therapy.
Developed by Thoratec, the device received a European CE Mark for both short-term and long-term support in October 2015. St Jude Medical acquired Thoratec in the same month. Abbott added the tool to its portfolio after acquiring St Jude Medical in January 2017.
A pre-market approval application for the device was submitted to the US Food and Drug Administration (FDA) for short-term cardiac support in advanced heart failure patients in December 2016, while FDA approval was received in August 2017.
The device received additional approval for long-term cardiac support as a destination therapy for patients with advanced heart failure in October 2018, based on the data from the MOMENTUM 3 clinical trial.
The FDA approved an alternative, less-invasive surgical method for implanting the HeartMate 3 in patients in January 2020.
In December 2020, the FDA approved an updated labelling of the device for use in paediatric patients with advanced refractory left ventricular heart failure.
More than 30,000 people globally have received Abbott’s life-saving heart pump to overcome advanced heart failure.
HeartMate 3 heart pump design and features
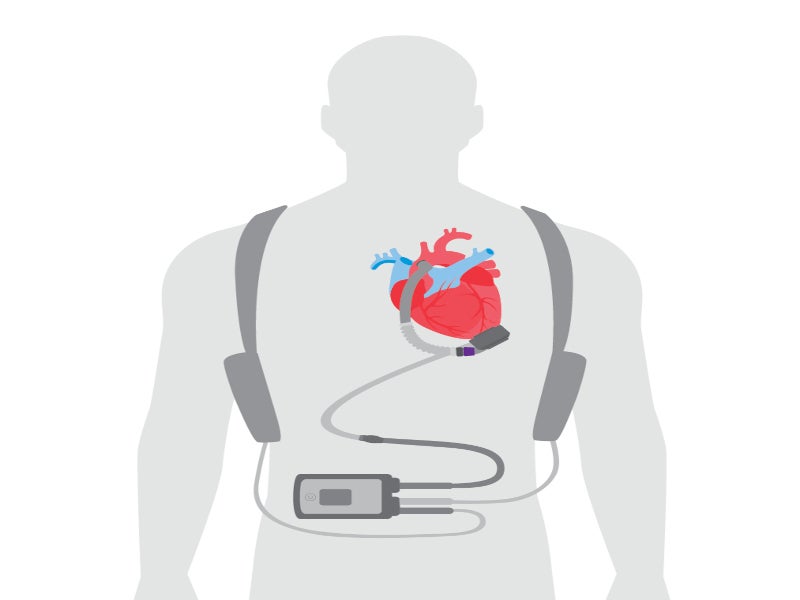
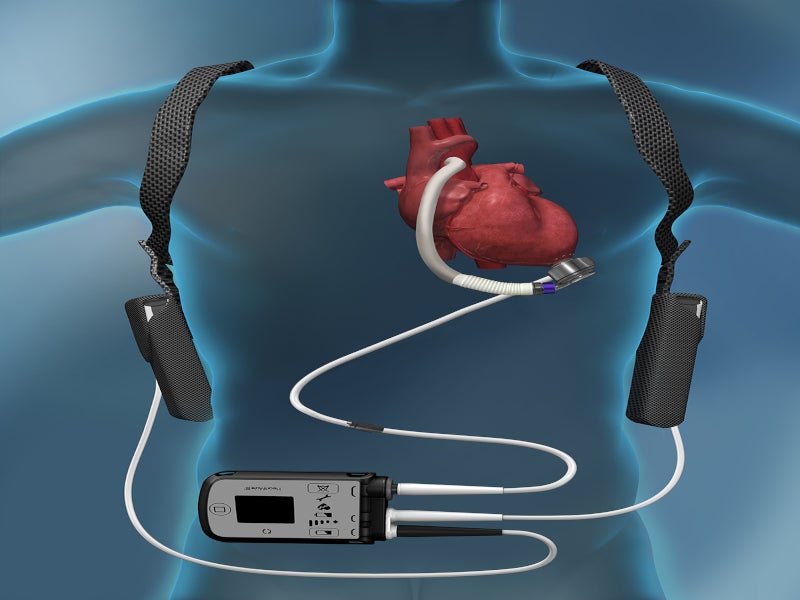
The HeartMate 3 LVAD is an implantable circulatory support system comprising an LVAD pump, system controller and battery unit.
The LVAD pump is situated in the thorax of the patient, covered with a titanium shell. The pump weighs 200gm, measuring 50.3mm in diameter and 55.8mm in height, including the inflow cannula connecting the pump to the patient’s circulatory system. The functioning voltage of the pump is from 10V to 17V, while power consumption is 4W.
The pump is connected to an external wearable system controller via a driveline that crosses the patient’s abdominal region. The system controller serves as a power and communication centre, providing information regarding the status of the pump and allowing clinicians to control and adjust its parameters.
The power cable connectors join external power devices such as a mobile power unit, power module or 14V lithium-ion batteries to the system controller.
HeartMate 3 heart pump technology details
The HeartMate 3 LVAD with Full MagLev Flow Technology fully magnetically levitates the flow, thereby reducing blood trauma passing through the pump and enhancing flow. HeartMate 3 is the first commercially approved Full MagLev flow technology-based centrifugal-flow LVAD.
It provides a fully levitated, self-centring rotor that eliminates the need for hydrodynamic or mechanical bearings. It features spacious, consistent blood flow pathways that decrease shear stress. Additionally, it incorporates intrinsic pulsatility, thereby reducing stasis and minimising the risk of thrombus formation.
Functioning of HeartMate 3 heart pump
In a healthy human, the left ventricle of the heart adequately pumps blood to the entire body. However, after surviving heart failure, the left ventricle gets weakened, failing to pump blood to the whole body.
The HeartMate 3 heart pump operates parallel to the heart and assists the weakened left ventricle in pumping blood to the aorta, increasing the blood flow mechanically. The pump can produce a blood flow rate of up to 10L/min.
Clinical trials on HeartMate 3 heart pump
The MOMENTUM 3 is the most extensive LVAD study in the world to determine patients’ need for short as well as long-term cardiac support. The study initially enrolled 294 patients and compared patient survival between the groups, using the HeartMate 3 LVAD and HeartMate II left ventricular assist system (LVAS).
Approximately 86% of the patients implanted with the HeartMate 3 LVAD showed six months of survival without any stroke or need for pump replacement, compared to 77% of patients using HeartMate II.
Among a total of 366 patients enrolled in the long-term clinical study, 77.9% using the HeartMate 3 LVAD showed event-free survival for two years without any stroke or need for pump replacement, compared to 56.4% of the patients using HeartMate II.
At two years, the survival rate of the patients using HeartMate 3 was 82.8%. The full result of the MOMENTUM 3 clinical study demonstrated high event-free survival, low adverse event rates, and improved quality of life in patients using the HeartMate 3 device.
At five years, HeartMate 3 demonstrated an improved survival rate of 58% compared to 44% with HeartMate II based on data from more than 1,000 patients. In addition, patients who used the HeartMate 3 had a reduced rate of adverse events such as stroke and bleeding, compared to patients who used the HeartMate II.
Further data from the study in August 2022 showed that the HeartMate 3 heart pump can extend a patient’s survival to five years or more, with the five-year survival rate for the patients reaching about 60%, which is rapidly approaching the five-year survival rates observed in heart transplant recipients with comparable risk profiles.
The MOMENTUM 3 study highlights the substantial survival advantages offered by Abbott’s heart pump technology, especially among patients facing limited therapeutic alternatives.
Furthermore, in November 2023, new data from the ARIES-HM3 clinical trial demonstrated that for patients with advanced heart failure, a HeartMate 3™ LVAD regime without aspirin in their antithrombotic regimen, including vitamin K antagonists (VKA), was non-inferior compared to a regimen incorporating aspirin. The omission neither escalates the risk of thromboembolism nor raises concerns, and correlates with a decrease in bleeding incidents.