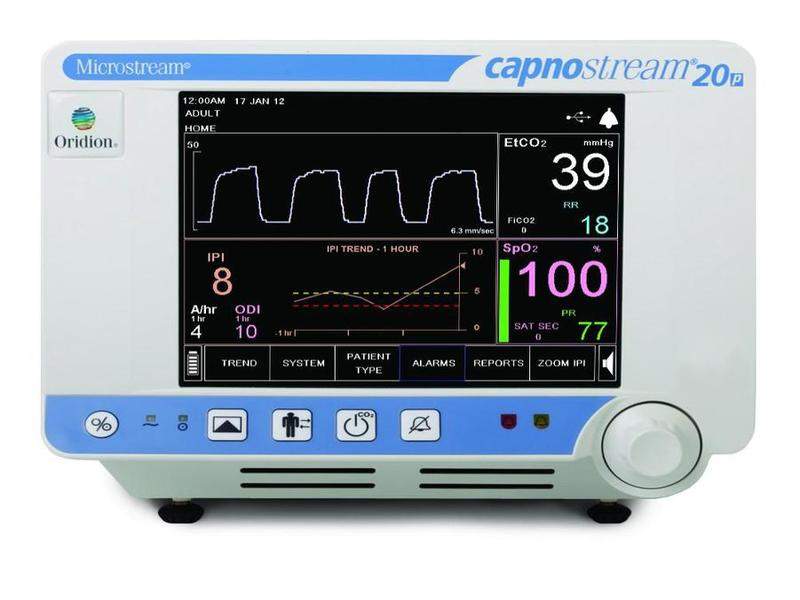
Medtronic has commenced a new clinical study called PRODIGY to detect high-risk opioid-induced respiratory depression (OIRD) patients, using its Capnostream 20p and 35 devices.
The prospective, multi-centre, post-market, international cohort study will assess the clinical and economic benefits of using pulse oximetry and capnography in 1,650 patients at 16 clinical centres.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Pulse oximetry is a device designed to measure oxygen saturation and pulse rate, while capnography is a tool used to measure respiration rate and carbon dioxide in exhaled air.
Capnostream integrates both pulse oximetry and capnography, and will be used in PRODIGY to create a device that will calculate the risk of OIRD in patients being treated with opioids for post-surgical or non-surgical pain in hospital wards.
The patients will be continuously monitored for any risk of respiratory depression.
Medtronic Respiratory and Monitoring Solutions and Early Technologies businesses senior vice-president and president Vafa Jamali said: "Information is powerful and the ability to identify patients at risk of respiratory compromise, who could benefit from continuous capnography and oximetry monitoring, may improve patient safety throughout the hospital."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Capnostream monitor will be used to collect data for end-tidal carbon dioxide (etCO2) and oxygen saturation (SpO2).
The continuous monitoring of carbon dioxide and oxygen levels is expected to enable the detection of other causes of respiratory compromise in patients on the general care ward.
A final data collection for the trial’s primary outcome is expected in February next year and the analysis is estimated to be completed a month later.
Image: Capnostream 20p monitor. Photo: courtesy of Medtronic plc.





