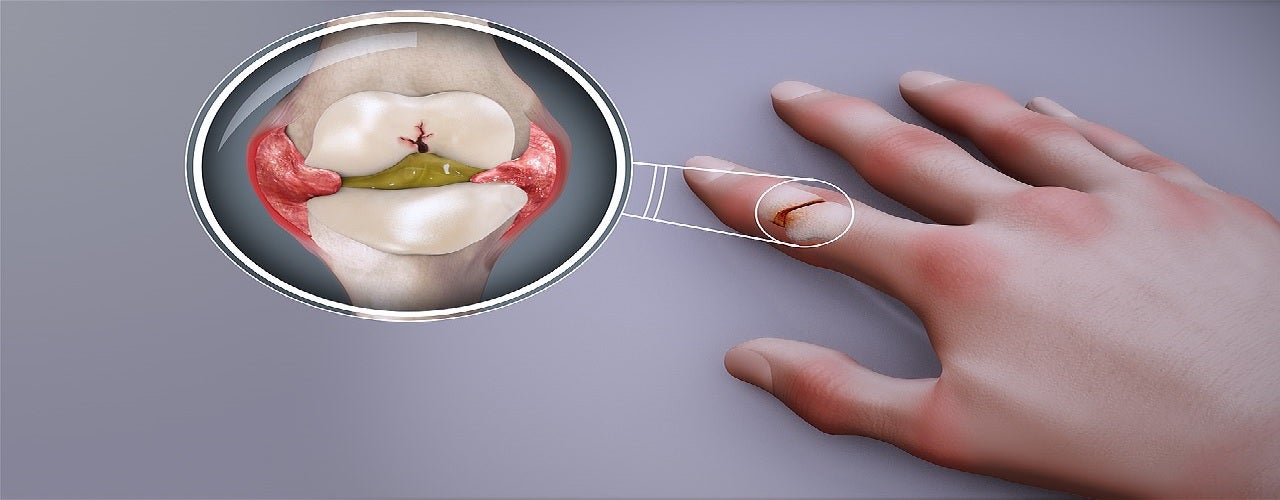
SetPoint Medical has secured breakthrough designation from the US Food and Drug Administration (FDA) for its new bioelectronic device.
The company has initially developed the device for the treatment of patients with rheumatoid arthritis (RA) who have a partial response to or are intolerant to more than one biologic drug.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It stimulates the vagus nerve to trigger the endogenous inflammatory reflex to reduce inflammation.
The company plans to expand the platform to include the treatment of chronic, inflammation-mediated autoimmune diseases in the future.
SetPoint Medical president and CEO Murthy V Simhambhatla said: “This is a significant milestone for SetPoint that will enable interactive communication with the FDA, priority regulatory review for the US market, as well as support reimbursement and patient access upon FDA approval.
“We look forward to initiating our pivotal trial in RA and working collaboratively with the FDA to advance the development of our novel platform.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIntroduced by the FDA in 2018, the breakthrough devices programme aims to provide patients and health care professionals with timely access to medical devices that enable more effective treatment over currently available products.
The FDA has granted Investigational Device Exemption (IDE) approval to SetPoint Medical for a multicenter, double-blind, randomised, sham-controlled pivotal trial for its bioelectronic platform.
The study will enrol up to 250 patients at 40 clinical trial sites across the US.
It will examine the safety and effectiveness of the SetPoint bioelectronic platform in patients with moderate-to-severe RA who are incomplete responders or are intolerant to biologic or targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs).
Approximately 1.5 million Americans are estimated to be diagnosed with RA. Many of these patients either do not respond, lose therapeutic response or are intolerant to biologic and targeted agents.



