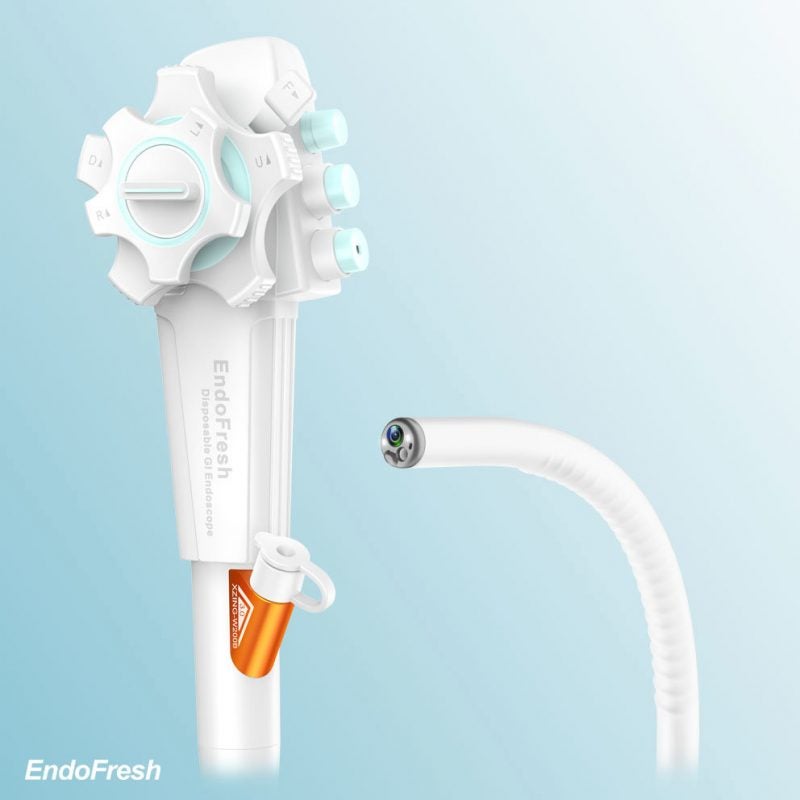
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Chinese company EndoFresh’s Disposable Digestive Endoscopy System for gastrointestinal (GI) purposes.
The device consists of a camera system with an all-in-one design, disposable upper GI endoscope and disposable colonoscope.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The system can be used along with the medical display and other peripheral devices to aid doctors in visualising, analysing and operating gastrointestinal endoscopy.
The latest device is intended to alleviate the cross-contamination problems caused by devices that are difficult to clean, as well as provide a safe design without needing reprocessing.
EndoFresh CEO Dr Lee said: “With this novel system, medical practitioners could offer patients a secure experience, which is available anytime and anywhere.
“It helps to prevent the risk of cross-infection and minimise the workload in preoperative screening and postoperative disinfection.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company noted that the risks associated with a disposable endoscope are low or negligible when compared with high acquisition and repair costs, expenses on washing equipment and cleaning staff.
A disposable system will also help avoid various points of failure linked to disinfection methods of a reusable endoscope.
In a separate development, the FDA has granted approval to market UroViu’s Uro-G, a flexible, disposable cystoscope.
The device’s deflectable tip facilitates interventional and diagnostic urologic surgeries in any room, anytime, without requiring reprocessing.
This could help practices to enhance their cystoscopic abilities and throughput without the need for capital investments or service contracts.
The per-procedure cost of owning and using a UroViu device is said to be low when compared to standard reusable devices.
NYU Grossman School of Medicine urology clinical assistant professor Jed Kaminetsky said: “Early adopters of UroViu’s pioneering technology will value the practicality of this safe, user-friendly and effective option, both for practice and patients.”
Kaminetsky added that the off-the-shelf availability of Uro-G is efficient and convenient for outpatient diagnostic procedures and stent removals.





