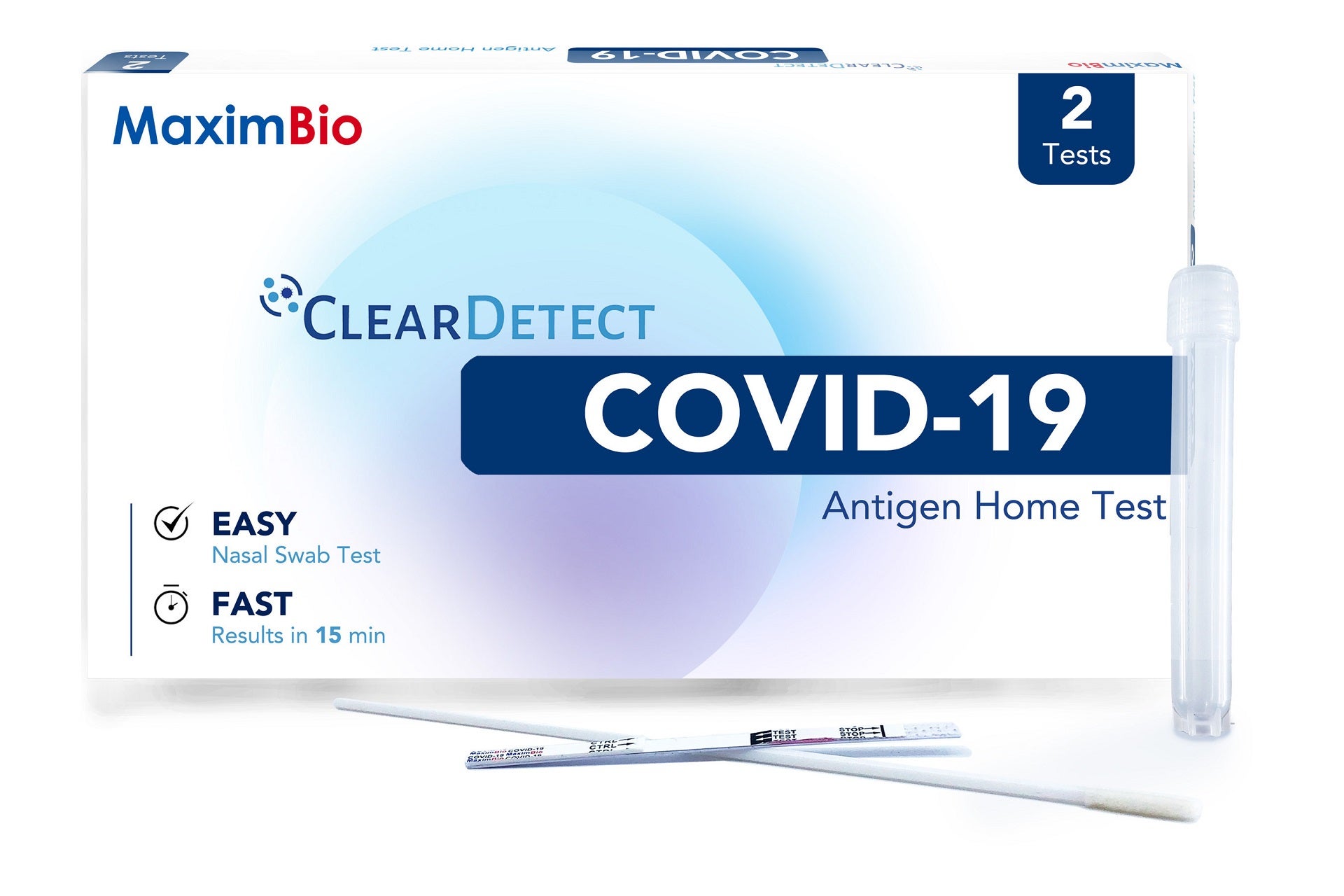
Diagnostic healthcare company Maxim Biomedical (MaximBio) has signed a marketing and distribution agreement with Thomas Scientific for its ClearDetect COVID-19 Antigen Home Test.
The antigen test received Emergency Use Authorisation (EUA) from the US Food and Drug Administration (FDA) this month.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is designed to detect SARS-CoV-2 proteins from nasal swab samples and provide results within 15 minutes.
The test uses Lateral Flow Assay (LFA) technology and can be performed easily at home without requiring equipment or a reader.
The ClearDetect COVID-19 Antigen Home Test involves only three components, specifically, a swab, test strip and test tube that comes pre-filled with sample buffer.
MaximBio stated that the test achieved 86.9% positive agreement and 98.9% negative agreement in comparison to a EUA authorised PCR method.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt detected 100% of live virus Omicron samples when assessed by the National Institutes of Health (NIH) with positive specimens for the Omicron variant.
The antigen home test detected the variant at a higher dilution compared to the other two EUA-approved antigen tests in the same study.
MaximBio chief commercial officer Anthony Morrison said: “Their dedication to provide the best products with personal, efficient service makes them an ideal partner for MaximBio as we team up to help meet the growing demand for Covid-19 testing.”
The ClearDetect COVID-19 Antigen Home Test will be available in two kit formats, either a two test pack for at-home use or bulk 25-test pack for healthcare settings, workplaces, schools and other high-volume testing scenarios.
Under the deal, Thomas Scientific will offer both kit formats and plans to price them competitively.
Furthermore, MaximBio is increasing its production to meet Covid-19 testing demand.





