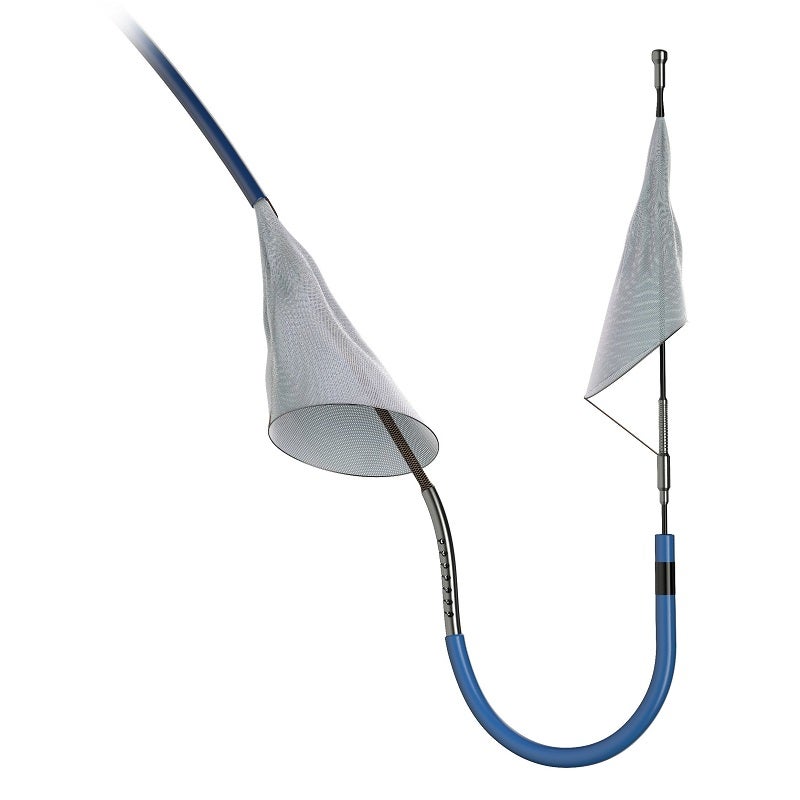
Boston Scientific has reported data from the PROTECTED TAVR clinical trial of the SENTINEL Cerebral Protection System.
The SENTINEL device has been designed for capturing and removing the embolic debris stemming from transcatheter aortic valve replacement (TAVR) before it reaches brain and causes a stroke.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The PROTECTED TAVR trial is claimed to be the largest randomised TAVR trial to date.
About 3,000 participants from more than 50 global sites were enrolled in the study.
In the trial, the participants were randomised into 1:1 ratio, where a group of patients were protected with SENTINEL device during TAVR, and the other group with TAVR alone.
It assessed the periprocedural stroke reduction and neurologic results in aortic stenosis patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company stated that the SENTINEL device did not meet the study’s primary endpoint, as the device showed 21% relative risk reduction through 72 hours after TAVR procedure or hospital discharge.
A secondary analysis showed significant 60% risk reduction of disabling stroke in patients treated with the SENTINEL device through 72 hours or time of hospital discharge.
Boston Scientific global chief medical officer Dr Ian Meredith said: “Considering strokes are unpredictable, can occur regardless of an individual’s clinical background and often take a great toll on a patient’s quality-of-life and financial stability, we believe the data appear to demonstrate a consistent effect from CEP technology across all patient populations in the trial – supporting the use of the SENTINEL device as an effective therapy to reduce the risk of the most debilitating form of stroke for patients undergoing TAVR.
“We look forward to additional data on this technology such as from the currently enrolling PROTECT TAVI trial in the United Kingdom, which will similarly evaluate TAVR-related stroke reduction using the SENTINEL device.”
The SENTINEL device was shown to be safe and effective in the previous clinical trials conducted in over 3,500 patients.





