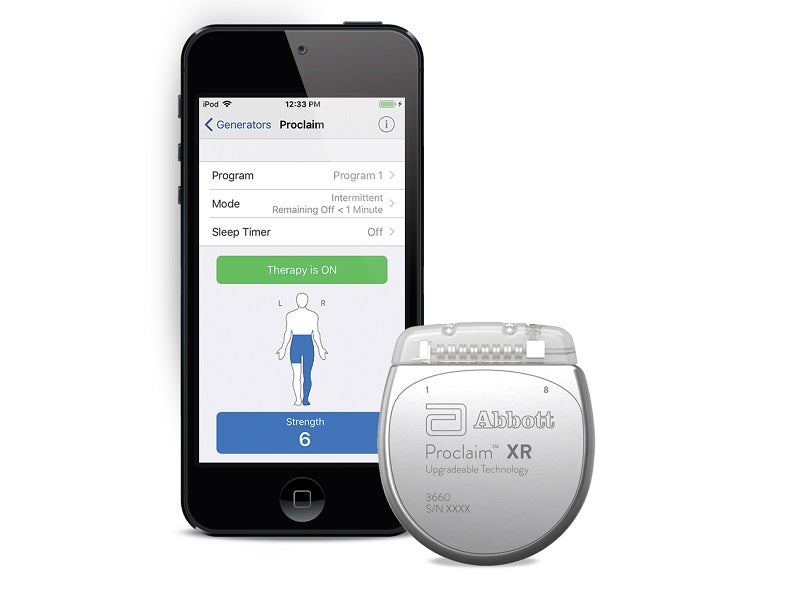
The US Food and Drug Administration (FDA) has granted approval to Abbott’s Proclaim XR spinal cord stimulation (SCS) system for the treatment of painful diabetic peripheral neuropathy (DPN).
In 2019, the Proclaim XR SCS system was approved for the treatment of chronic pain in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The latest approval expands the system’s indication to offer chronic pain relief to DPN patients through low stimulation doses.
The Proclaim XR SCS system can offer DPN patients pain relief, as they often require alternatives to traditional oral medication treatments.
It comes with a battery that lasts up to ten years, while other SCS systems require frequent charging sessions to continue therapy.
After completion of a minimally invasive trial, patients will be implanted with the Proclaim XR SCS device.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThey will then be able to control their therapy through an Apple device.
Abbott neuromodulation vice-president Pedro Malha said: “As a leader in diabetes care, Abbott is intimately familiar with the challenges people with diabetes encounter daily.
“This new indication for Proclaim XR will drive meaningful change in the treatment of pain associated with diabetic peripheral neuropathy and will be an important tool for physicians and patients in managing this debilitating condition.”
DPN is a complication of diabetes that is characterised by damage to the nerves running to the feet.
According to the company, nearly 50% of diabetic adult patients will develop DPN during their lifetime, with symptoms including pain and numbness in the legs, feet and hands.
Currently, there are no disease-modifying treatments available for DPN, only symptom management and behavioural modifications to stop further nerve damage, which can be caused due to high glucose levels.
Users of the Proclaim XR SCS system can also use Abbott’s NeuroSphere Virtual Clinic technology.
NeuroSphere Virtual Clinic is a remote patient care technology that facilitates communication between physicians and patients, allowing them to receive treatment adjustments remotely.





