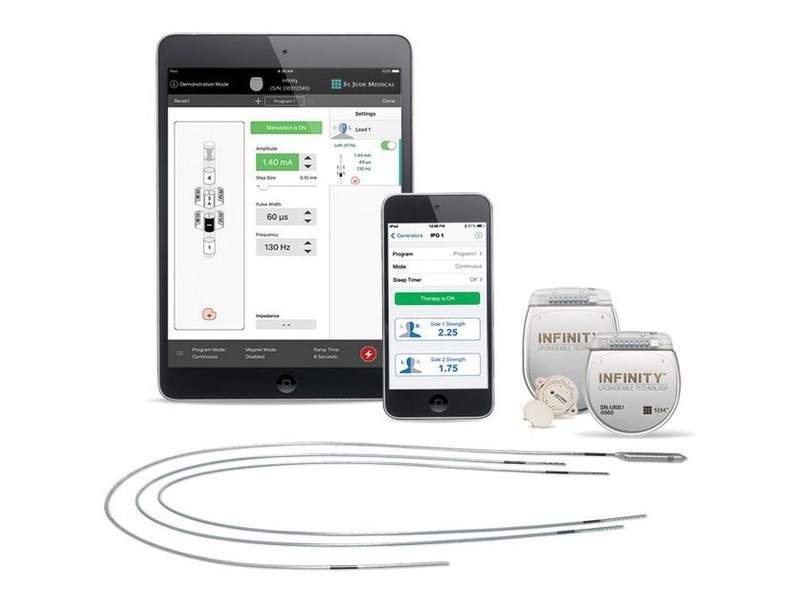
The US Food and Drug Administration (FDA) has granted approval to Abbott to upgrade the software of its Infinity Deep Brain Stimulation (DBS) System designed to treat patients with Parkinson’s disease and essential tremor.
The over-the-air upgrade is set to add magnetic resonance (MR)-conditional labelling and various advanced features to the DBS device.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Before the upgrade, patients using DBS therapy could not access these features due to the potential requirement for an MRI in the future.
The approved labelling will allow the use of the improved Infinity device, which is said to be the first FDA-cleared MR-conditional directional DBS system.
Existing implanted Infinity systems can be upgraded through secure Bluetooth wireless technology, eliminating the need for surgery.
Abbott movement disorders medical director Binith Cheeran said: “With this software upgrade, Abbott delivers on a promise to develop powerful features that strengthen the Infinity DBS system’s already patient-centric platform, which uses familiar Apple technology and frees the patient from recharging their device.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We are committed to continued innovation in neuromodulation, developing ongoing advancements and efficiencies for physicians and, most importantly, helping thousands of people who are battling movement disorders live fuller lives.”
The Infinity DBS system comprises directional leads that send stimulation to target areas of the brain in order to enhance patient outcomes and minimise side effects.
Abbott designed the device to support any capability upgrades and the addition of new therapy features via simple, over-the-air updates.
The system also has the European CE-Mark, along with approvals in around 30 other countries such as Russia, Saudi Arabia and Taiwan.





