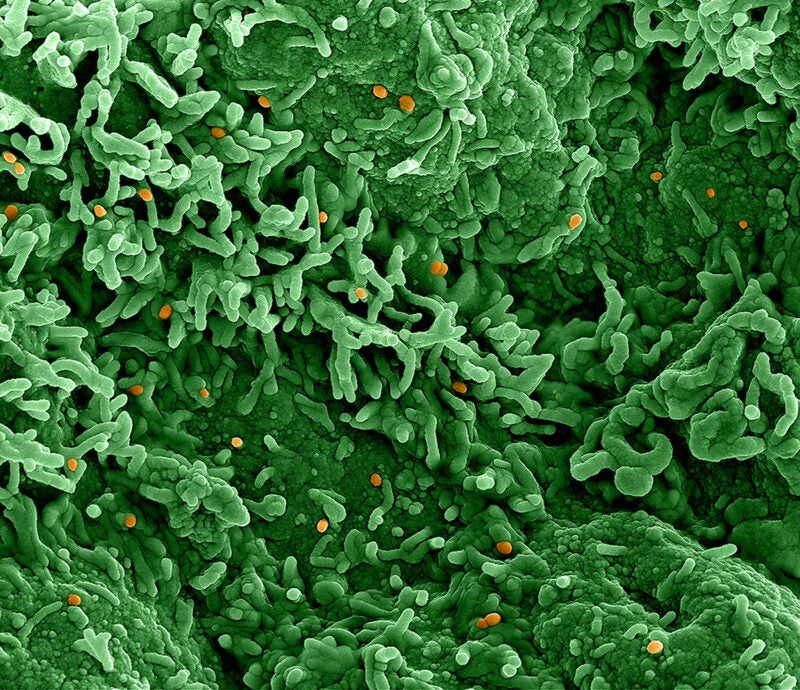
Applied DNA Clinical Labs (ADCL), the clinical laboratory subsidiary of Applied DNA Sciences, has submitted a validation package to the New York State Department of Health (NYSDOH) for its monkeypox virus test, the Linea Monkeypox Virus 1.0 Assay.
The PCR-based test has been designed to detect the genetic signature of the Clade II variant of the monkeypox virus, which is now prevalent in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is part of ADCL’s infectious disease testing offering, which centres on diagnosing infectious diseases that can reoccur.
The company stated that the Linea Monkeypox Virus 1.0 Assay would represent its second laboratory-developed test (LDT) in less than one year if granted approval by the NYSDOH.
Upon receiving approval, ADCL plans to carry out monkeypox testing at its clinical laboratory evaluation program (CLEP)/clinical laboratory improvement amendments (CLIA) authorised molecular diagnostics laboratory in Stony Brook, New York, US.
The Linea Monkeypox Virus 1.0 Assay will use proven workflows for accurate results.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataApplied DNA president and CEO Dr James Hayward said: “Our pursuit of monkeypox testing via LDT combines what we believe to be the quickest regulatory path for diagnostic approval with our proven ability to deliver short turnaround time-to-results that, as Covid-19 has taught us, can help contain virus spread.
“Upon NYSDOH approval, ADCL has the testing capacity to deploy to help keep New Yorkers safe.
“Moreover, as the test kit manufacturer, we maximise the control over our own supply chain to ensure both quality and availability.”
In January, the company’s Linea 2.0 COVID-19 Assay LDT received conditional approval from the NYSDOH.
During the same month, ADCL unveiled plans to deploy its Linea 1.0 COVID-19 Assay for the rapid detection of mutations, indicating the presence of the BA.2 subvariant (BA.2) of Omicron, in Covid-19-positive samples.





