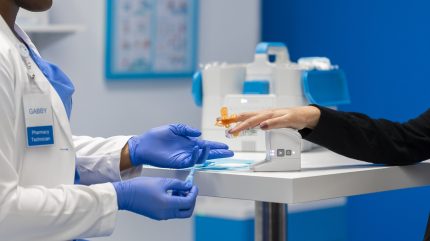
Babson Diagnostics has developed a hand-warming device that aims to improve the collection of capillary blood samples from a fingertip.
The Babson Hand Warmer device, within the BetterWay blood testing ecosystem, has completed the validation testing.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Babson said it collaborated with experts in biomedical engineering to create a device that could effectively warm the hand, a technique known to facilitate blood collection from a fingertip.
The BetterWay system is claimed to reimagine blood testing by integrating Babson’s proprietary sample preparation and laboratory technologies with the BD MiniDraw Capillary Collection System, which is a result of a strategic partnership between Babson and Becton, Dickinson and Company (BD).
Slated for a commercial launch within the year, the Babson Hand Warmer simplifies blood testing and enhances accessibility. It requires only a small amount of blood from a finger to deliver medically accurate results.
Babson research and development vice-president Roy Barr said: “The Babson Hand Warmer supports blood collection from the finger, providing simultaneous warming of the hand with collection to create a repeatable and more pleasant blood testing experience for the customer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The device is another example of how BetterWay is putting the needs of the customer first.”
The hand-warming device was developed in collaboration with the University of Texas (UT) at Austin Department of Biomedical Engineering.
A validation study was conducted on the device with staff at pharmacy partners to ensure compliance with the regulatory standards of the US Food and Drug Administration (FDA).
In a press statement, Babson said: “The device received overwhelmingly positive feedback from pharmacy staff trained as collection techs and the customers getting a BetterWay blood test.”
The Babson Hand Warmer is registered as a Class II medical device with the FDA.
In 2022, Babson and BD expanded their collaboration to advance blood sample collection into new care settings.





