BrioHealth Solutions has commenced subject enrolment in the US for the randomised INNOVATE trial, which will assess the BrioVAD system in treating refractory left ventricular heart failure.
The controlled, prospective, non-blinded, non-inferiority, multi-centre trial aims to assess the effectiveness and safety of the system.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is set to determine if patients living with the system spend less time in the hospital compared to those with the standard of care left ventricular assist device (LVAD).
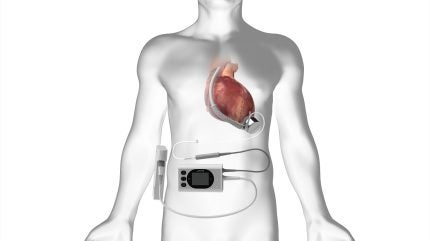
Featuring the fully magnetically suspended BrioVAD Pump, the BrioVAD System is a full-support, durable ventricular assist device designed to enhance the quality of life in patients.
It also includes uniquely engineered external components and has been under development since 2008.
More than 350 non-Americans were treated with ventricular assist systems incorporating the ‘BrioVAD Pump technology’.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe pump’s magnetic bearing design allows for a smaller pump size with a larger impeller, decreasing surgical invasiveness.
Additionally, the BrioVAD Pump’s driveline design, which electrically connects the pump to external components, results in a thinner and more flexible driveline. This feature may potentially lessen driveline-associated infections.
The external patient-worn components of the system have been structured to improve user experience with only two components, potentially improving patient quality of life.
The BrioVAD Pump’s construction and large diameter impeller combination facilitate an improved blood flow pathway design within the pump. This design is expected to improve device hemocompatibility and hemodynamics, thereby reducing the critical complication risk.
BrioHealth Solutions CEO Chen Chen said: “We are thrilled to kick off the INNOVATE trial, following a phenomenal journey of innovation, engineering, and quality refinement to bring the BrioVAD System to life.
“Despite advancements in ventricular assist devices, there remains a pressing need for improved device performance and patient outcomes, and BrioHealth is committed to addressing this gap.”





