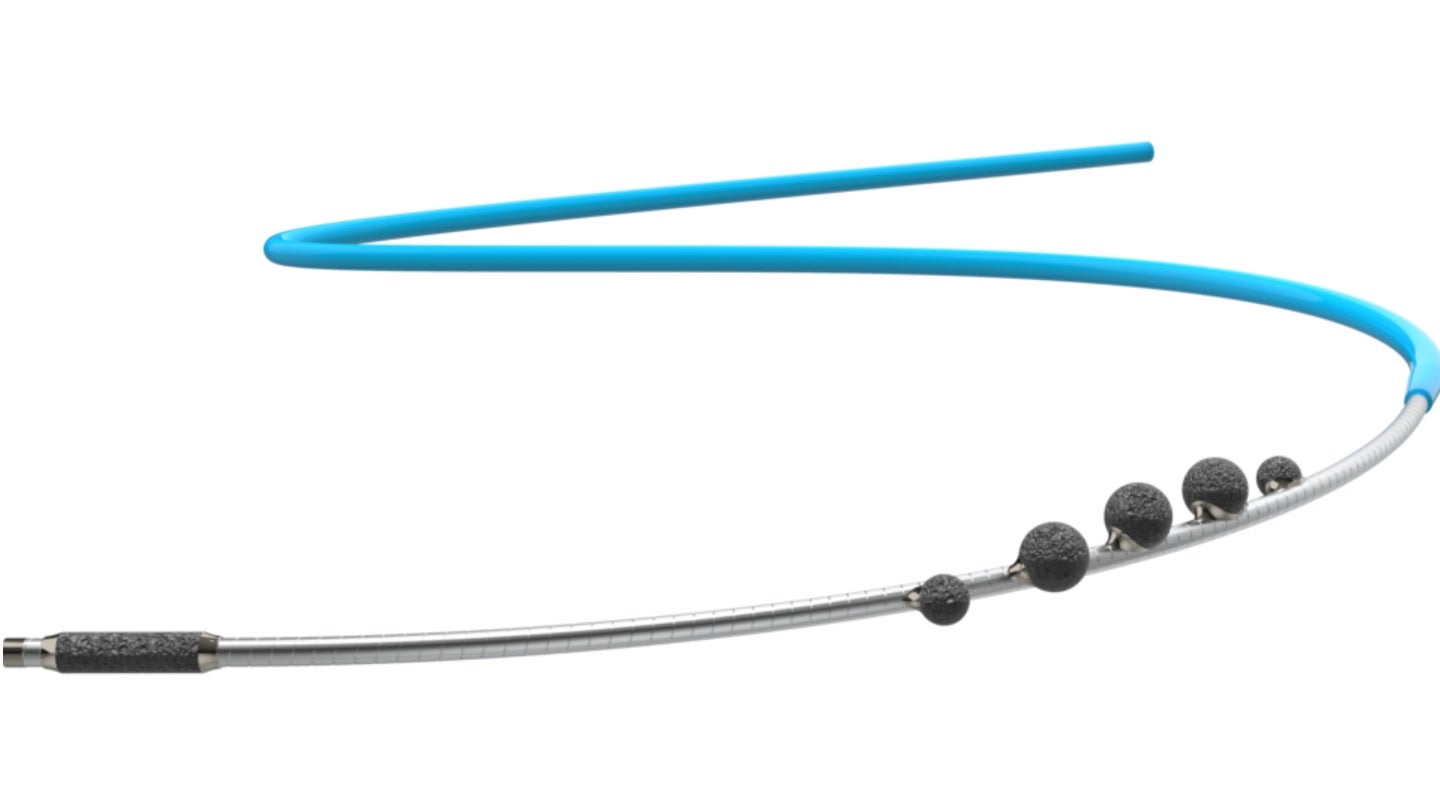
Cardio Flow has obtained 510(k) clearance from the US Food and Drug Administration (FDA) for its FreedomFlow Orbital Atherectomy Peripheral Platform.
The platform deploys a modern action mechanism for clearing plaque blockages in the arteries of the legs.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Leveraging angular momentum physics, the catheter-based design enables the formation of a spiral geometry that places five diamond-coated spheres in simultaneous contact with the vessel wall, whether advancing or retracting.
A tip coated with a diamond makes it easier to navigate the driveshaft through narrow blockages.
This approach allows physicians to treat complex peripheral artery disease (PAD) flexibly across a broad spectrum of vessel sizes, ranging from 2mm in the ankle to 8mm in the hip.
It also enhances their ability to treat multiple arteries and blockages within the same vessel using a single device.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCardio Flow CEO Michael Kallok said: “Cardio Flow is committed to providing meaningful solutions that directly address the needs of physicians and their PAD patients through innovative product development.
“Many of the existing atherectomy devices on the market have various design constraints and capital equipment costs.
“With FreedomFlow, we strove to provide physicians with the freedom to treat complex PAD hip to heel with a simple yet sophisticated device that would answer the call for flexible treatment options and cost savings for healthcare systems.”
Cardio Flow is focused on the design and development of minimally invasive peripheral vascular products.





