Endologix has announced 24-month results from the trial of its Detour System for fully percutaneous femoropopliteal bypass procedures.
The company recruited 202 patients in Europe and the US for the DETOUR 2 study.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The single-arm, international and multicentre trial was designed to assess the new Detour System and demonstrated that 96% of enrolled patients had chronic total occlusions, with 32.7cm mean lesion length.
DETOUR 2 also achieved 100% technical success for treated patients. The primary safety endpoint was exceeded with a 7% rate of major adverse events in 30 days.
The study also revealed that the freedom from CD-TLR at 24 months was 76.7%, while secondary patency was 82.3%.
At 24 months, the freedom from symptomatic DVT was 96.5% and the freedom from major lower limb amputation was 98.5%.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
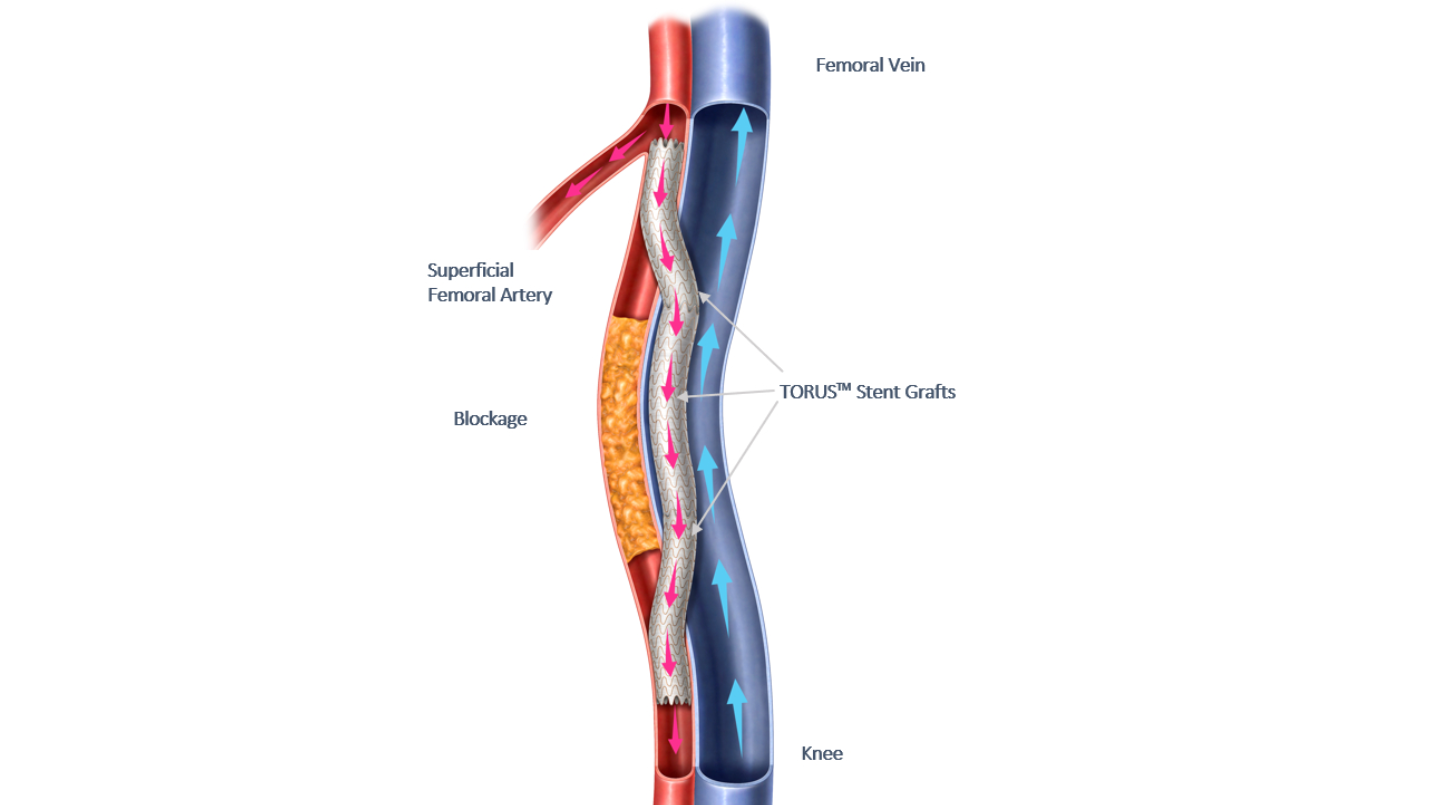
By GlobalDataEndologix designed the Detour System to provide a unique approach to treating peripheral arterial disease (PAD).
It allows physicians to percutaneously bypass lesions in the superficial femoral artery by utilising stents that are routed through the femoral vein for restoring blood flow to the leg.
Endologix president and CEO professor Matt Thompson said: “We are delighted to present the two-year results of the DETOUR 2 Study which investigates the use of the PTAB therapy in patients with very long SFA lesions.
“The results suggest that the DETOUR System offers a viable approach in patients where open surgery is the currently recommended treatment. We are excited to see more patients benefit from this unique approach to the treatment of complex PAD.”
The Detour system includes the ENDOCROSS device and TORUS stent grafts.





