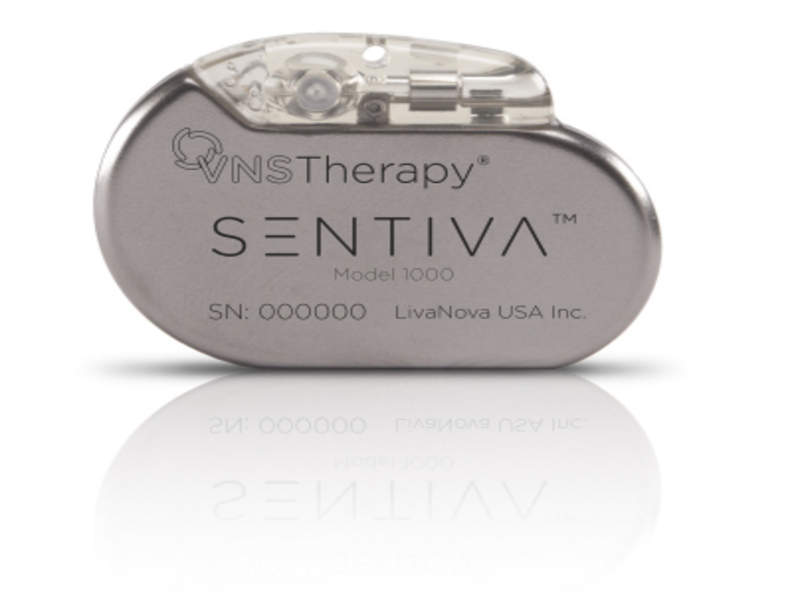
UK-based firm LivaNova has obtained approval from the US Food and Drug Administration (FDA) for its new Vagus Nerve Stimulation (VNS) Therapy System designed to treat drug-resistant epilepsy.
The new system features the SenTiva implantable generator and next-generation VNS Therapy Programming System, which comprises a wireless wand and new user interface on a small tablet.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Intended to deliver physician-directed customisable therapy, the smart technology device is said to have demonstrated the ability to minimise the number and duration of seizures.
LivaNova North America president and Neuromodulation business franchise general manager Jason Richey said: “We created SenTiva and the accompanying VNS Therapy Programming System based on feedback received from patients and physicians to ensure ease of use, better patient care, and cost-effectiveness.
“In addition, SenTiva’s compact size allows for a more comfortable experience for paediatric patients, which is beneficial now that VNS Therapy is the first and only system that is FDA-approved for drug-resistant epilepsy in children as young as four years of age.”
SenTiva comes with a detect-and-respond mode to prevent seizures and for automatic delivery of extra therapy to stop them if they start.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe device is designed to collect and log seizure-associated events such as the patient’s body position and heart rate variations.
Compatible with SenTiva and other LivaNova VNS Therapy generators, the VNS Therapy Programming System provides advanced guided programming to allow quick treatment with a single touch.
The system also offers scheduled programming, which enables safe prearrangement of different steps during one visit to a physician’s office, resulting in a gradual and automatic increase of therapy without the need for repeated visits.
Day and night programming of the system is intended to facilitate customisation of the therapy based on the patient’s needs.





