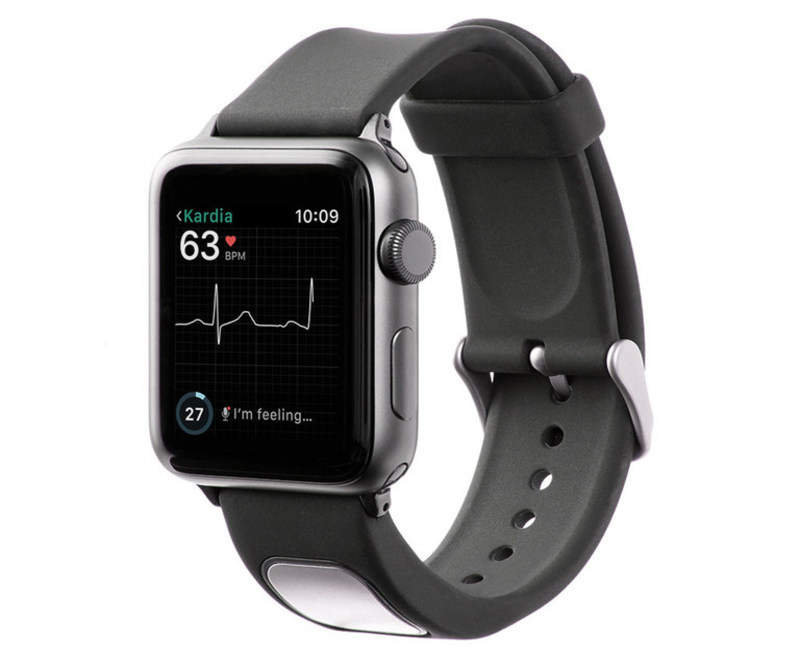
The US Food and Drug Administration (FDA) has cleared electrocardiogram (EKG) technology developer AliveCor’s medical device accessory, KardiaBand, for Apple Watch.
KardiaBand is designed to allow Apple Watch users to capture EKG for the rapid detection of normal sinus heart rhythms and atrial fibrillation (AFib).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The medical device accessory can also record an EKG in 30 seconds upon touching an integrated sensor, and data from the Kardia App is displayed on the watch.
In addition, the firm has launched the new SmartRhythm feature within the Kardia app for continuous monitoring of the correlation between the heart and physical activities.
The feature leverages artificial intelligence and inputs from the watch’s heart rate and activity sensors, and alerts the users to capture an EKG when heart rate and activity are out of sync.
AliveCor CEO Vic Gundotra said: “KardiaBand paired with SmartRhythm technology will be life-changing for people who are serious about heart health.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“These capabilities will allow people to easily and discreetly check their heart rhythms when they may be abnormal, capturing essential information to help doctors identify the issue and inform a clear path of care to help manage AFib, a leading cause of stroke, and other serious conditions.”
The firm uses advanced artificial intelligence along with mobile, cloud and micro-electrode technology to deliver cardiac care through easy, quick and inexpensive detection and management of potential abnormal heart rhythms.
KardiaBand and SmartRhythm on Apple Watch are currently available through the firm’s subscription service.





