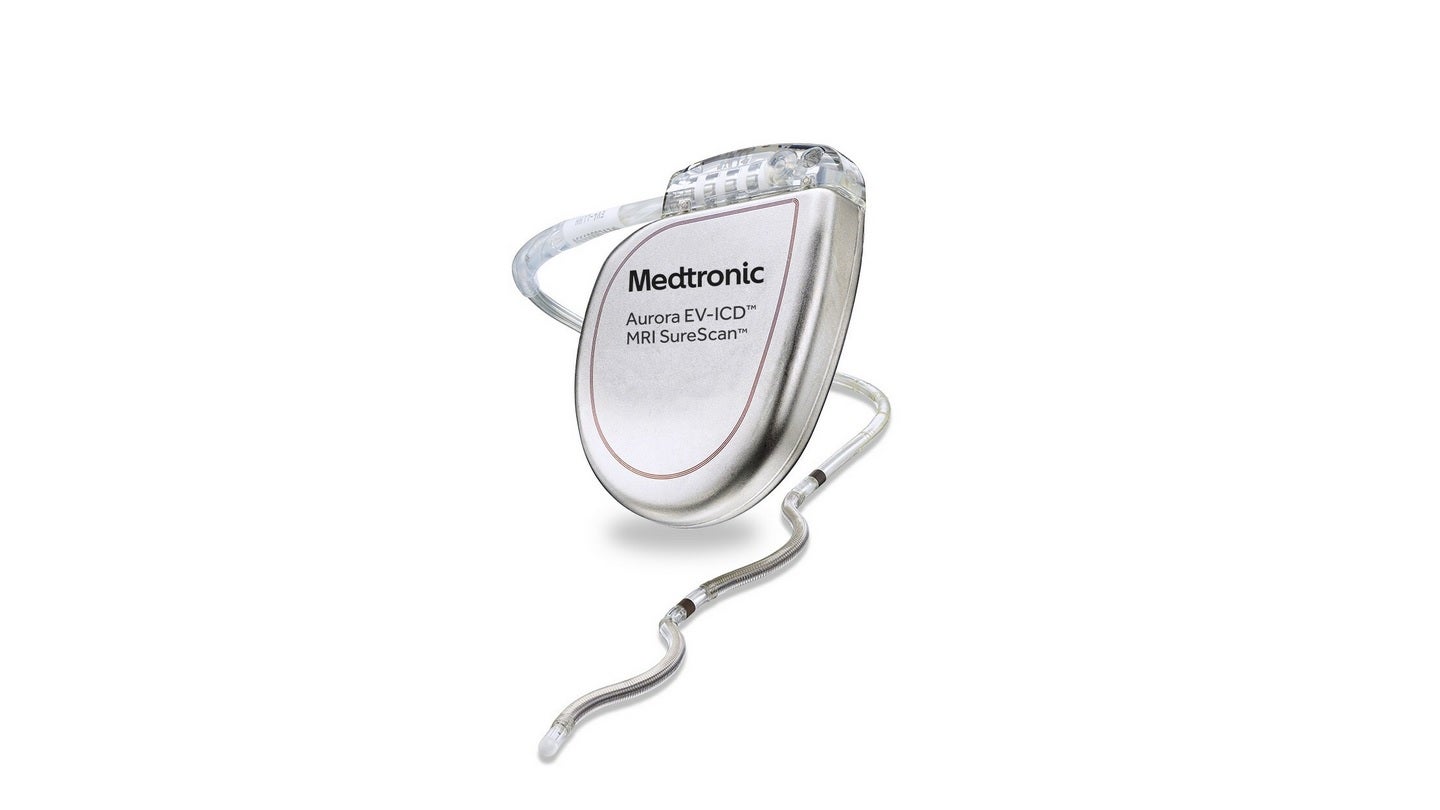
The US Food and Drug Administration (FDA) has approved Medtronic’s Aurora EV-ICD MRI SureScan extravascular implantable cardioverter-defibrillator and Epsila EV MRI SureScan defibrillation lead for the treatment of fast heart rhythms that may cause sudden cardiac arrest.
Aurora EV-ICD approval encompasses the system’s exclusive implant tools.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The approval is based on the favourable results of a global pivotal trial demonstrating the system’s effectiveness and safety.
It is claimed to be the first-of-its-kind device that delivers the benefits of traditional, transvenous ICDs with a lead (thin wire) positioned below the breastbone, outside of the heart and veins.
The system offers defibrillation, backup (pause-prevention) pacing and anti-tachycardia pacing (ATP) therapies through a device that is similar in shape, size and longevity to traditional, transvenous ICDs.
The Aurora EV-ICD system is implanted beneath the left armpit and the lead is placed beneath the breastbone using a minimally invasive technique, unlike traditional ICDs.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTo avoid certain complications associated with transvenous leads, the Epsila EV defibrillation lead is placed outside of the heart and veins.
Medtronic Cardiovascular Portfolio’s cardiac rhythm management business chief medical officer Alan Cheng said: “ICDs remain the gold standard for prevention of sudden cardiac death and while the subcutaneous ICD avoids certain complications associated with transvenous defibrillators, it has limitations that may affect a patient’s comfort and quality of life.
“With the Aurora EV-ICD system, patients can benefit from the only ICD placed outside the vascular space that provides ATP and backup pacing in a device that is nearly half the size and with 60% greater projected battery longevity compared to the competitor’s subcutaneous ICD.”
The Aurora EV-ICD system will soon become available for limited commercial availability in the US.





