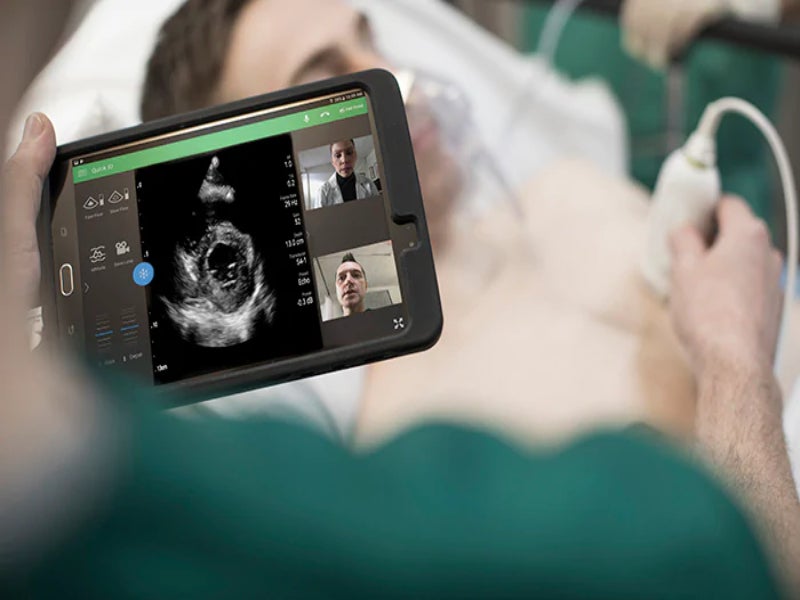
Royal Philips has received 510(k) clearance from the US Food and Drug Administration (FDA) to market its ultrasound solutions for the management of lung and cardiac complications related to Covid-19.
The clearance applies to the company’s ultrasound systems such as the EPIQ series, Affiniti series, Lumify, CX50 and Sparq diagnostic ultrasound systems and to off-cart solutions, including QLAB Advanced Quantification Software.
Following the regulatory clearance, Philips will now offer detailed, practical guidance to support clinicians, using its systems and software for patients affected by Covid-19.
The new guidance highlights the specific presets, transducers, quantification tools and other capabilities that are offered on Philips’ ultrasound systems relevant in evaluating and managing lung and cardiac complications.
Philips Ultrasound general manager and senior vice-president Bich Le said: “Many healthcare providers have told us that our handheld and portable ultrasound solutions are playing a valuable role in their efforts to combat Covid-19.
“With this regulatory clearance, we can offer clear guidance to ensure safe and effective use of ultrasound to manage Covid-19-related lung and cardiac complications. At the same time, we are investing significantly to ramp up production globally, including at our ultrasound manufacturing plants in the US.”
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe company noted that ultrasound has proved to be effective in imaging peripheral lung tissue affected by pneumonia, which is closely associated with Covid-19 lung complications.
Covid-19 patients are considered to be at increased risk for cardiac complications as respiratory strain can also cause cardiac dysfunction.
A cardiac ultrasound exam can help in analysing the effects that disease progression may have on heart function.
By imaging patients at the point of care such as in the Emergency Department (ED) or Intensive Care Unit (ICU), clinicians can diagnose and monitor patients without the need to move them around the hospital.
It also reduces the risk of virus transmission to other patients and healthcare professionals.