US-based orthopaedic device manufacturer, Forma Medical has launched the first minimally invasive surgery (MIS) solution for hammer toe – OptimalHT.
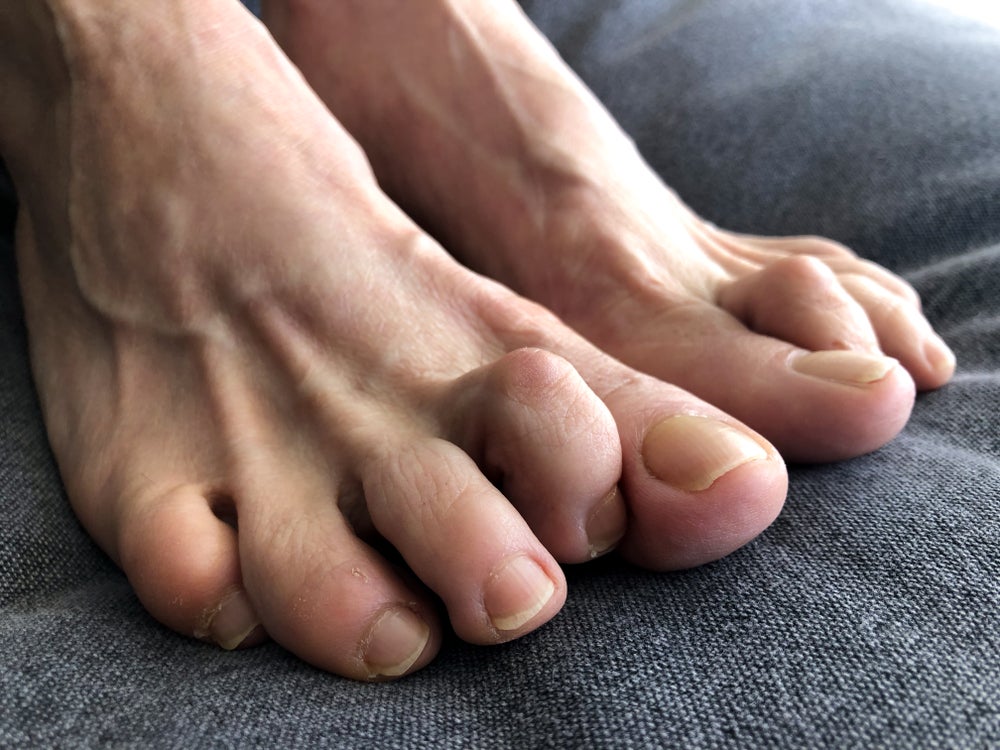
Hammer toe is a condition that causes the second, third or fourth toe to bend at the middle, resembling a hammer. The condition is commonly caused by wearing short narrow shoes that constrain the foot and can be treated early on without surgery but if left unattended it can require a surgical procedure.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Forma Medical say OptimalHT provides surgeons with a MIS solution to correct the condition. James Gault, Vice President of Forma Medical said: “The instrumentation guides wire placement through the three target bones which is the most difficult part of the procedure. This is extremely challenging to do without our hammer toe-specific instruments and typically requires a large incision to do so accurately.”
He added: “Our entire system has been designed specifically for hammer toes – everything from the implants to the drivers and the unique targeting instrumentation. There is no other system on the market that has instrumentation to complete hammer toe correction surgery in a minimally invasive fashion.”
The launch of OptimalHT follows its US Food and Drug Administration (FDA) approval in July.
Gault said: “With this FDA approval, we now have a platform to launch life-changing systems across multiple anatomies. We are continuing to push the envelope and expand our portfolio to advance patient care.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“It’s no secret that minimally invasive surgery is the future of Foot and Ankle care,” said Dr. Noman Siddiqui, DPM at LifeBridge Health, Baltimore added: “The novel OptimalHT approach for digital correction is ideal for less invasive surgery in the foot to reduce edema, expedite recovery, and achieve the results my patients demand.”
According to GlobalData, the orthopaedic devices market is expected to reach nearly $50 billion in 2023. One of the trends contributing to the market value is robotic assisted surgeries. A GlobalData report indicates the market for orthopaedic robotic surgical systems and accessories is expected to reach $984 million globally in 2023, with year-over-year growth of 25.6%.
In the US, the number of orthopaedic devices is expected to grow from 15,535,269 in 2015 and is forecast to reach 23,808,557 in 2030.
OptimalHT will be debuted during the Orthopedic Foot & Ankle Society (AOFAS) 2023 annual meeting in late September.





