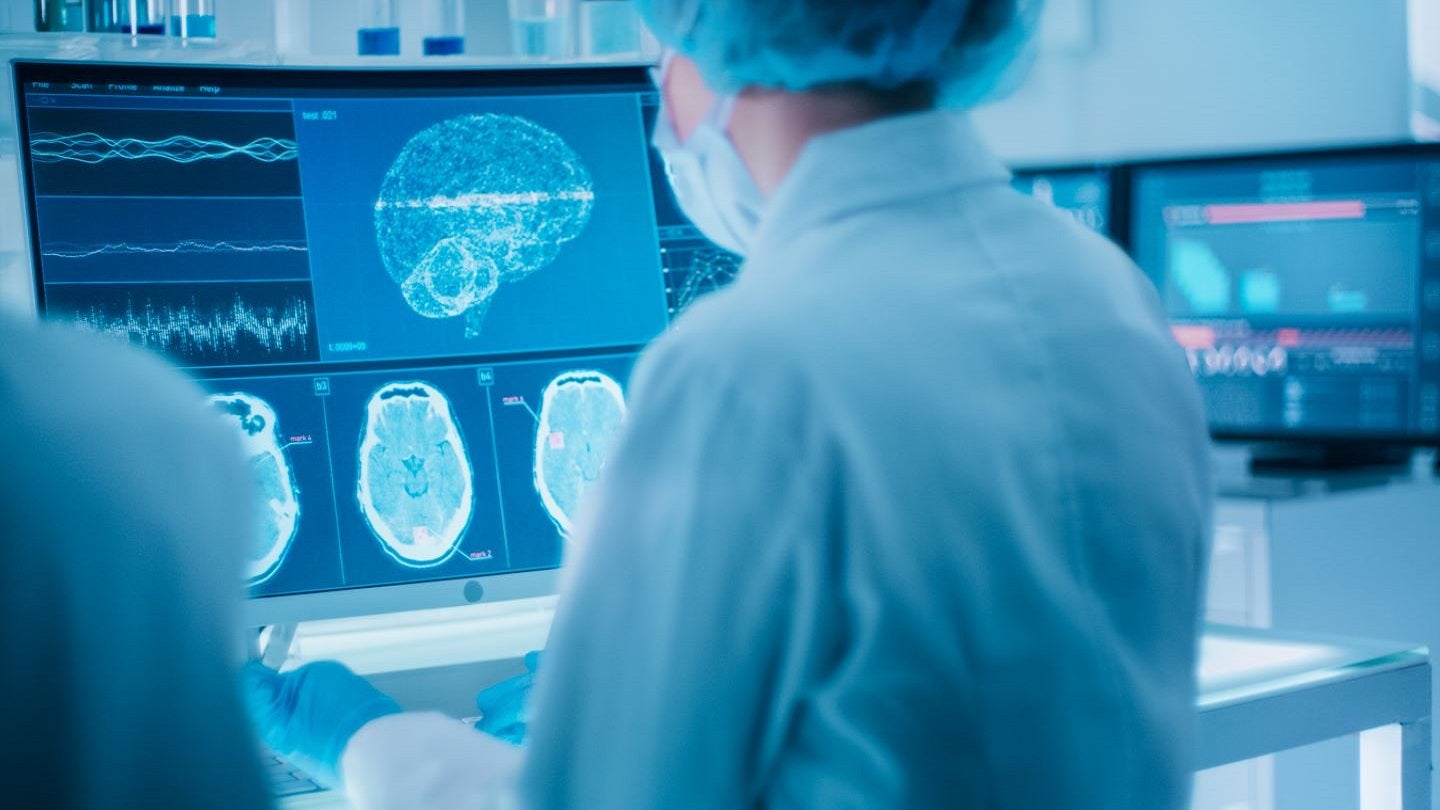
Imeka has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Advanced Neuro Diagnostic Imaging (ANDI) quantitative imaging software.
The ANDI-generated report offers crucial reference information on brain white matter to medical professionals, including neurologists and radiologists.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This software enables the extraction of white matter bundles, which link specific regions of the brain and perform microstructure analysis along these connections.
Imeka co-founder and scientific advisor Maxime Descoteaux said: “Imeka has spent the past 12 years developing novel neuroimaging technologies that play an influential role in the search for and development of cures for brain diseases.”
Leveraging modelling, tractography and fibre bundling algorithms, the device processes diffusion-weighted images for mapping microstructural properties of the white matter.
A DICOM-encapsulated PDF report will be generated by ANDI, focusing on bundles that exhibit a significant deviation from the normative range.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe report also provides a detailed analysis of the microstructural and macrostructural values of all bundles.
Imeka CEO Jean-René Bélanger said: “We are pleased to announce FDA 510(k) clearance of ANDI, our quantitative imaging software and make the technology available to healthcare providers across the US – which is going to have a major impact on brain disease management in the coming years.
“This also comes at a very crucial time with the announcement of the addition of two new CPT 3 codes by the AMA for quantitative brain MRI assessment, which we expect our clients to be able to get reimbursement from, starting in January 2024.”





