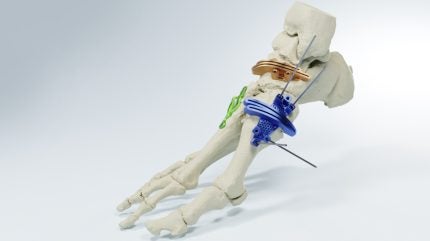
MedCAD has received 510(k) clearance from the US Food and Drug Administration (FDA) for its AccuStride Foot and Ankle System.
This clearance permits the company to provide surgeons with patient-specific precision devices designed to correct multiple foot and ankle pathologies in a single operation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
MedCAD said the patent-pending AccuStride System represents its initial venture into the lower extremities market.
The company already produces custom-designed implants for cranial and maxillofacial reconstruction, including surgical devices and virtual planning.
MedCAD CEO and president Nancy Hairston said: “MedCAD’s newly announced, patient-matched solution is unlike anything else available for surgeons who routinely perform complex or revision cases in the treatment of the entire foot.
“We have experienced a surge in inquiries and interest from leading foot and ankle orthopaedic specialists who are eager to use our ‘whole foot’ solutions for multiple pathologies, and we expect these custom 3D-printed devices to reduce the frequency and duration of surgeries, and to deliver high-quality, durable outcomes.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMedCAD’s AccuStride System aims to improve surgical outcomes for proximal phalanx and metatarsal arthroplasty, as well as offers custom solutions for Lapidus revisions and corrections of cavus foot.
The system’s components are made from UV-curable acrylate polymers or titanium alloy materials.
These components can be delivered within five days following approval of medical imaging and surgeon design. The system can be used in patients aged 12 and above.
Hairston added: “The goal of MedCAD’s portfolio of patient-matched product solutions is to provide surgeons with customisable options that are as anatomically unique as their patients.”
In December 2024, MedCAD began using 3D printing technology to produce facial reconstruction plates.
These plates are designed to address a range of surgical needs and complexities with a shorter delivery window.
Available in various thicknesses, from 2mm to 2.8mm, the plates include features such as inferior border hooks, graft trays, and allowances for standard mesh between the plate and mandible.





