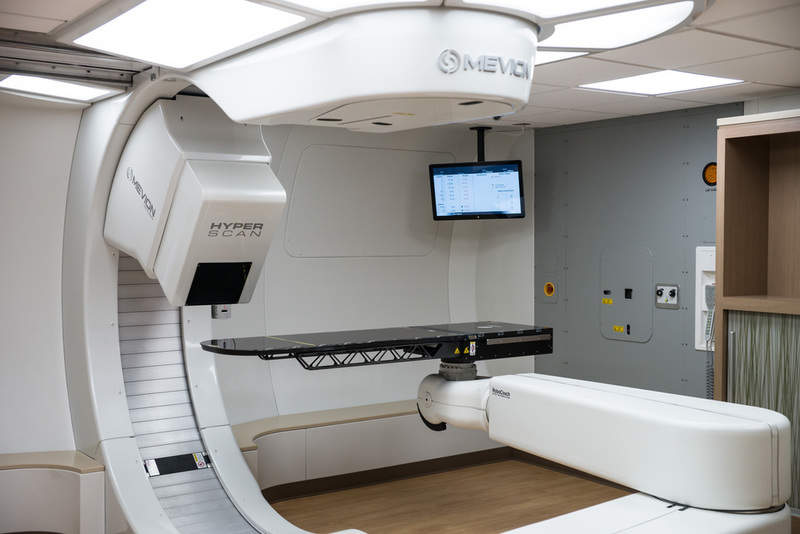
Mevion Medical Systems has obtained CE-Mark for its MEVION S250i proton therapy system, allowing clinical use of the system in the European Union (EU) and countries that recognise the mark.
MEVION S250i is a compact, fully integrated platform that comprises gantry-mounted superconducting synchrocyclotron, a treatment couch and advanced in-room IGRT imaging.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The therapy system features a pencil beam scanning (PBS) technology, Hyperscan, with fast energy layer switching and optimised spot sizes to enable rapid and sharp proton treatments.
Hyperscan also includes the Adaptive Aperture proton multi-leaf collimator system for precise dose gradient, minimising dose uncertainty at the edge of a tumour and to prevent unnecessary radiation to healthy tissues or sensitive areas.
Mevion Medical Systems EMEA Business Development field vice-president Perjan Pleunis said: “This milestone is part of Mevion’s global expansion of cutting-edge compact proton therapy systems in Europe.
“Mevion has led the way in establishing compact proton therapy as the most technologically advanced and financially viable choice in proton therapy.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMEVION S250i is intended to reduce cost per patient by decreasing capital and operating costs, as well as increasing treatment throughput through linac-like workflow and low-maintenance requirements.
The first MEVION S250i proton therapy system is scheduled to be installed by next year at the Maastro Clinic’s Zuid-Oost Nederland Protonen Therapie Centrum (ZON PTC) in the Netherlands.
MEVION S250i is part of the firm’s MEVION S250 Series of high-powered proton therapy treatments, which leverage Direct Dose beam delivery technology.





