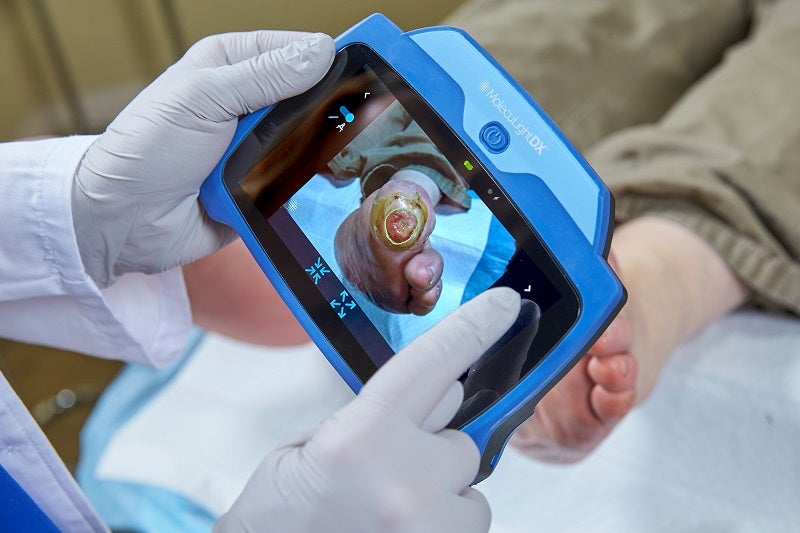
Canadian medical device company MolecuLight has secured a group purchasing agreement with the US-based AllSpire Health GPO for wound imaging devices.
Under the terms agreed, MolecuLight’s i:X and DX point-of-care wound imaging devices will be available to the members of AllSpire.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
AllSpire aggregates the purchasing volumes and expenses and streamlines supplier negotiations across the supply chain to help health systems optimise their operations.
MolecuLight’s imaging devices are claimed to be the only devices of their kind to have received US Food and Drug Administration (FDA) approval.
They allow clinicians to visualise the presence, location and load of bacteria present in wounds in real-time.
Findings from a 14-site clinical trial involving 350 patients demonstrated that the clinical standard of care alone detected 15% of wounds, while the use of MolecuLight devices led to a 400% improvement in wound detection.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMolecuLight CEO Anil Amlani said: “We are thrilled to have entered into a supply contract with AllSpire Health GPO.
“Through the i:X and DX, we hope to enable significant cost-savings and improvements in clinical outcomes.
“AllSpire’s extensive member base can now easily access the MolecuLight wound imaging devices and see the clinical benefits in their wound care practices.”
MolecuLightDX is designed for detecting the presence and location of elevated bacterial loads, as well as digital measurement of the wound.
The company’s i:X device allows clinicians to identify more wounds that contain elevated bacterial burden and measure them at the point of care.
Both devices provide bacterial information at the point of care that can help make clinical decisions and provide targeted wound therapies.
AllSpire Health GPO strategic sourcing senior director James Wallick said: “We are most impressed with the clinical utility that the MolecuLight i:X and DX devices provide to wound care professionals and are pleased to offer the MolecuLight portfolio via our Group Purchasing Agreement to our member hospitals.”





