US-based medical device maker Novian Health has secured CE-Mark approval to market its Novilase Breast Therapy in the European Union (EU) and Switzerland.
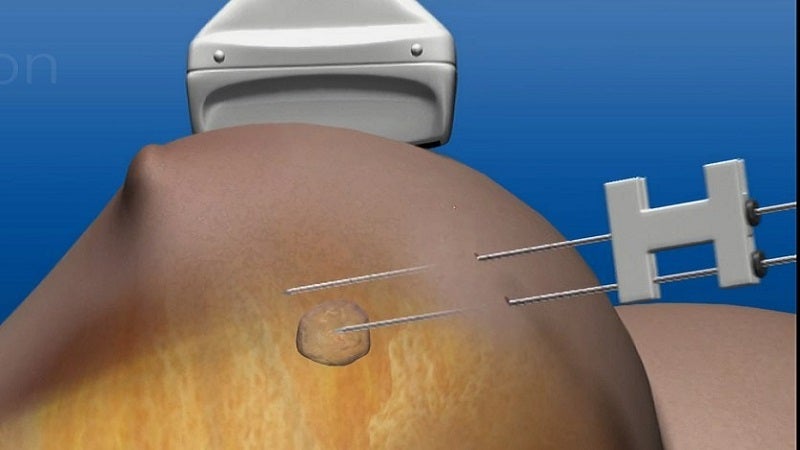
Novilase is a thermal ablation device that delivers laser induced heat for focal destruction of breast tumours up to 2cm in size.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
During a minimally invasive procedure, two small probes are inserted into the breast and guided to the tumour using ultrasound imaging.
One of the probes uses heat to destroy the tumour, while the second monitors the procedure via temperature. The non-surgical therapy is completed within 30 minutes and its success can be confirmed with follow-up imaging.
Novilase has been designed for patients with early stage breast cancer and benign solid breast tumours called fibroadenomas.
Novian Health president and CEO Henry Appelbaum said: “The CE-Mark is a significant milestone for Novian Health, as Novilase is the first thermal ablation device approved for treatment of malignant breast tumours.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Laser therapy can help women to avoid the trauma and risks of surgery. We’re thrilled to have the opportunity to bring this option to women in the EU.”
In an open-label, multi-centre Phase II clinical trial in the US and UK, Novilase demonstrated the ability to completely destroy more than 90% of malignant breast tumours during a single procedure.
The company noted that trial participants reported better health-related quality of life outcomes when compared to lumpectomy surgery. In addition, the investigators did not observe any serious adverse events.
Novilase study principal investigator Barbara Schwartzberg said: “Using laser technology to treat breast tumours is less invasive, less painful, and produces better cosmetic results, in addition to being significantly less expensive. Compared to surgery, it’s simply a better technique all around.”
The technology has received US Food and Drug Administration (FDA) approval to treat fibroadenomas.
The regulator also agreed to an investigational device exemption (IDE) confirmatory study for supporting an application to market Novilase for focal destruction of malignant breast tumours.





