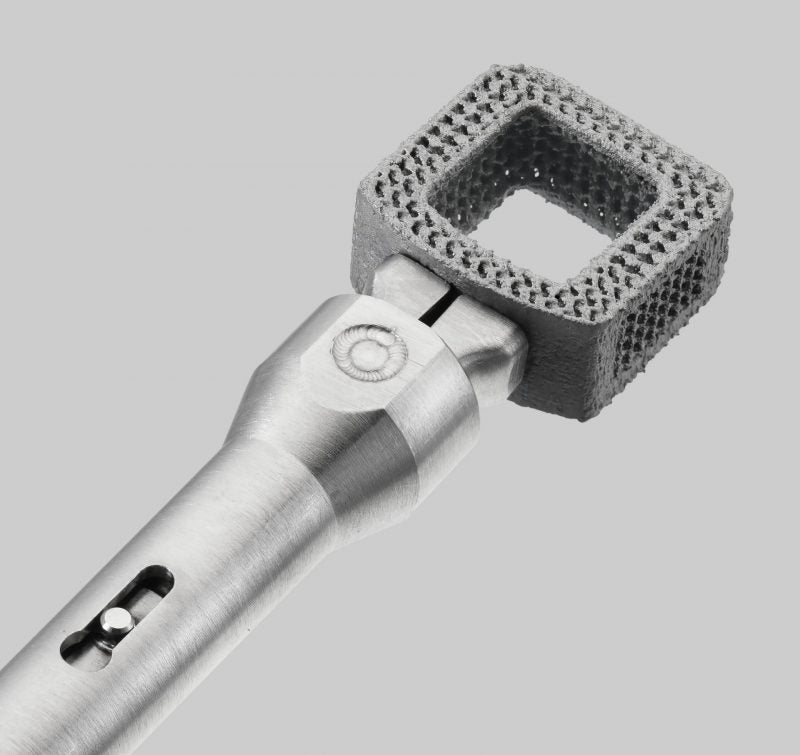
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Orthofix Medical’s 3D-printed CONSTRUX Mini Ti Spacer System, designed to facilitate anterior cervical discectomy and fusion (ACDF) procedures.
Made with nanoscale surface features, the CONSTRUX Mini Ti cervical spacer with nanovate technology has been implanted in the first patient.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The system has 3D-printed porous titanium with macro, micro and nanoscale surface features. In an in vivo ovine lumbar spinal fusion model, the nanoscale surface demonstrated an increase in proliferation and alkaline phosphatase activity in human stem cells in vitro.
The system also features endplates with 400-micron pores and 50% porosity designed to support bone ingrowth.
Furthermore, the functional gradient porous structure, with 80% porosity at the midline of the implant, facilitates better fluoroscopic visualisation.
Simple instrumentation allows the system to be implanted easily, while the large centre opening is designed with concaved inner walls for packing bone-grafting material.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOrthofix global spine president Kevin Kenny said: “Orthofix’s cervical spine offerings feature a wide array of implants ranging from motion-preserving products like the M6-C artificial cervical disc to advanced interbody and fixation solutions that aid surgeons in restoring spinal alignment and decreasing pain and nerve compression.
“We are dedicated to expanding our comprehensive cervical spine solutions with technologies like the CONSTRUX Mini Ti Spacer System that can make a meaningful difference in our product offerings and the lives of patients.”
This system is just one among many Orthofix products with nanotechnology FDA clearance. Others include the CONSTRUX Mini PTC Spacer System, the Pillar SA PTC Spacer System and the FORZA PTC Spacer System.
The implant’s 3D-printed endplates with nanovate technology show a significant improvement in growth factors linked to osteogenesis and osteoblast maturation when compared to solid polyether ether ketone (PEEK) devices, thereby providing a better osteogenic environment for bone ingrowth.
Last October, Orthofix launched a new O-GENESIS Graft Delivery System together with AlloQuent Structural Allograft Q-Pack, a ready-to-use form of cervical and lumbar spacers for allograft procedures.





