Netherlands-based PulseCath has received a CE mark under the EU Medical Device Regulation (MDR) for its mechanical circulatory support device, iVAC 2L.
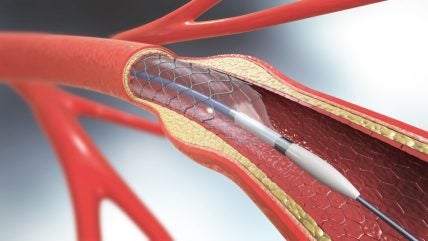
PulseCath’s iVAC 2L system is a percutaneous mechanical circulatory support (MCS) device. The system provides critical haemodynamic support during high-risk revascularisation procedures in cases of acute myocardial infarction and cardiogenic shock, as well as for high-risk patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The iVAC 2L system is inserted through the femoral artery, with the catheter inlet positioned in the left ventricle and the outlet valve is placed at the height of the ascending aorta. The external valve, when activated, acts as a circulatory backup by pumping blood from the left ventricle to the aorta and synchronising with the patient’s natural cardiac cycle. The device can be removed once the treatment is over.
The percutaneous MCS device is marketed in Greater China and selected in the Asia Pacific region by China-based Huadong Medicine. As part of the agreement, PulseCath received investment in its share capital and payment for regulatory and development activities. Furthermore, the company is eligible to receive royalties on sales in Huadong Medicine’s commercial area.
The cardiac assist device is expected to generate $446.9m in sales across the five major European markets, namely France, Germany, Italy, Spain and the UK, next year, as per a GlobalData market analysis report. The MCS device segment is predicted to pull in $175m during the same time across the five European markets.
Many companies are developing percutaneous cardiac assist devices. In September 2023, PercAssist completed its first patient case in its first-in-human clinical study investigating its percutaneous synchronised cardiac assist (PSCA) device in patients with chronic heart failure. The balloon catheter system is designed to provide haemodynamic support with minimally invasive placement and without the need for coagulants.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn June 2023, Israel-based Magenta Medical started an early feasibility study for its Elevate percutaneous left ventricular assist device (pLVAD) for high-risk percutaneous coronary intervention (HR-PCI). Being the world’s smallest heart pump, it provides temporary mechanical circulatory support during HR-PCI procedures.





