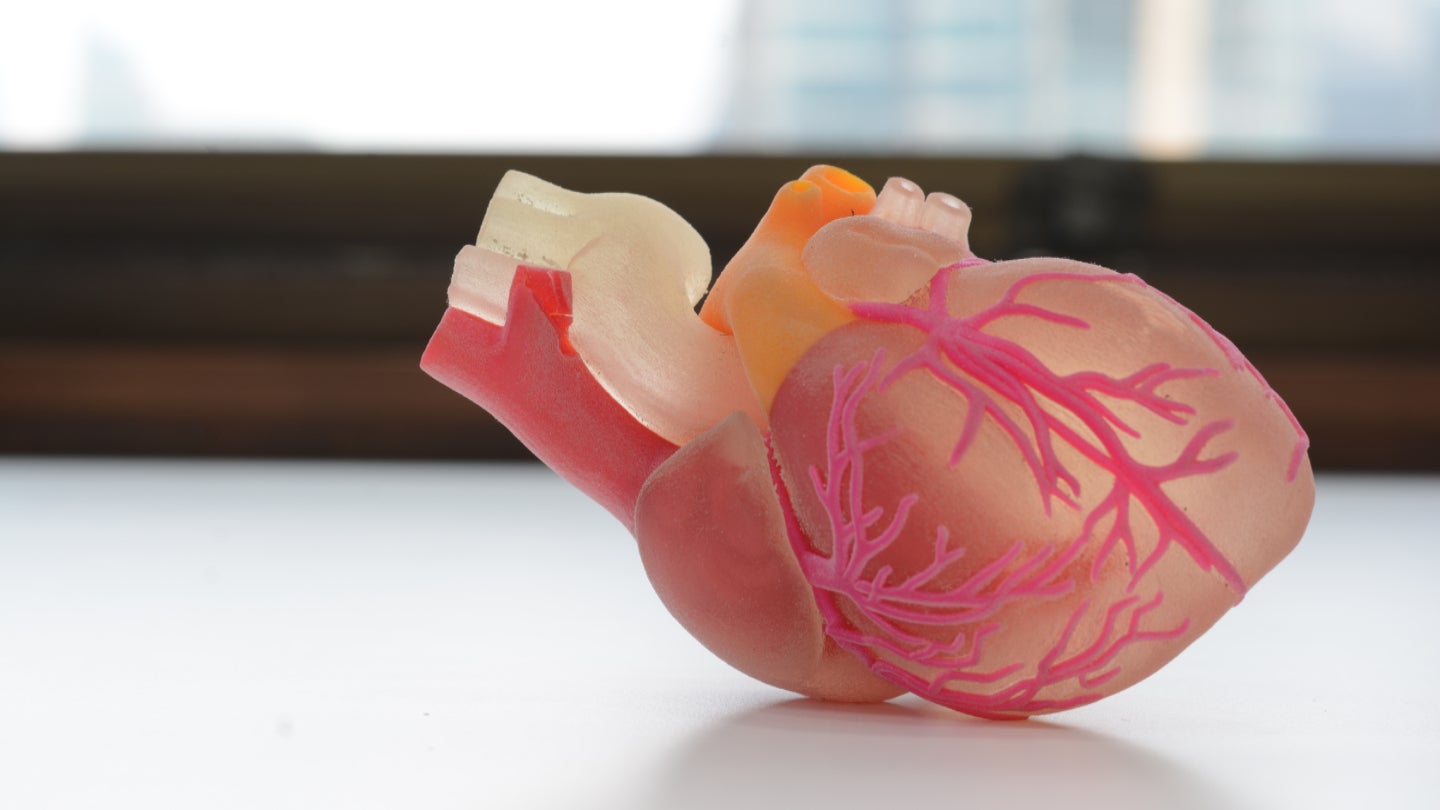
Japanese imaging and electronics company, Ricoh, has partnered with a Belgian 3D printing firm in a bid to produce 3D models of a patient’s anatomy.
Announcing the partnership as part of the Radiology Society of North America (RSNA) annual meeting on the 27th of November, the company will partner with Materialise to bring point-of-care 3D printing to hospitals across the US allowing for the production of aids such as anatomical models of a patient’s anatomy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Utilising Materialise’s software alongside Ricoh’s printing hardware, Ricoh says the deal will allow clinicians to easily request anatomical models of a patient’s anatomy, mapped digitally through imaging software, to better explain procedures and conditions to patients using patient-specific model.
Vice president of Materialise North America, Bryan Crutchfield, said: “Outside of large academic medical centres, physician and patient access to 3D printing applications has been limited.
“This is often due to a lack of resources and technical knowledge to implement and operationalize the technology in the hospital environment.
“This partnership with Ricoh brings a large managed services infrastructure, which will enable hospital systems to more quickly and affordably implement and scale 3D technology for their physicians and patients. We are excited to partner with Ricoh to bring our end-to-end software platforms to support 3D planning and 3D printing applications at the Point of Care.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataRicoh aims to use its technology to give clinical independence, allowing them to request and print objects for their own specific use on a case-by-case basis, in what the company calls ‘democratising’ the technology.
This approach allows Ricoh to sidestep the requirements for the company to be treated as an US Food and Drug Administration (FDA)-registered medical device manufacturer, avoiding some of the costs and regulations incurred through the process but also urges clinicians to be aware that items made using 3D printing could be considered medical devices once in use and would subject to regulation.
Research by GlobalData details how the 3D printing market saw sales of $19bn in 2022, with that figure forecasted to rise to $23bn by the end of 2023, continuing to rise to $36 billion by 2025.
Managing director of additive manufacturing for Ricoh, Gary Turner, said: “Materialise’s software tools will not only help Ricoh provide a better experience for its customers but also support Ricoh in its goal democratising equitable access to impactful tools such as patient-specific anatomic models.”
At the same time, the RSNA conference saw an announcement from Dutch firm, Phillips, introducing medical imaging devices that utilise artificial intelligence (AI) with the aim of reducing time loss among clinicians and introducing cloud-based storage.
Also, at RSNA, GE HealthCare is exhibiting more than 40 new innovations to address radiologist shortages, mounting workloads and a lack of access to (MRI)s.





