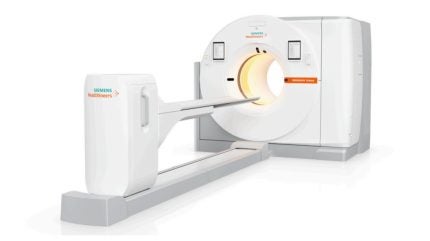
Medical device giant Siemens Healthineers has received US Food and Drug Administration (FDA) clearance for its positron emission tomography/computed tomography (PET/CT) scanner Biograph Trinion.
The scanner’s PET/CT platform features a new air-cooled, digital detector based on lutetium oxyorthosilicate (LSO) crystal elements. With a time of flight (TOF) of 239 picoseconds (ps), the Biograph Trinion is a single, integrated platform that includes PET, CT, and post-processing workflows on one system.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Siemens Healthineers has said that the integrated post-processing imaging applications include oncology, theranostics (a combination of diagnostics and therapeutics to identify and treat certain forms of cancer), cardiovascular imaging, and neurology imaging (including Alzheimer’s disease).
The scanner, which also features mood light features to help calm anxious patients, has a mobile workflow powered by AI and enabled by a portable Scan&Go tablet. An integrated patient camera allows the user to monitor and communicate with the patient.
In the announcement accompanying the clearance, James Williams, head of molecular imaging at Siemens Healthineers, said: “With the Biograph Trinion, Siemens Healthineers is proud to offer customers a high-performance PET/CT scanner that delivers the precision and speed needed for clinical demands. This new system is designed to be user- and patient-focused, as well as a sustainable investment in terms of reduced installation and operational costs and easy, on-site scalability.”
Last month, Siemens Healthineers announced a $250m investment in a new UK production facility aimed at manufacturing superconducting magnets to be used in magnetic resonance imaging (MRI) devices.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHowever, earlier this year, the company announced the closing of its Fast Track Diagnostics unit, which affected 90 jobs. The move is part of a restructuring effort, with the company blaming Covid-19 for the decline in diagnostics demand. In November 2023, Siemens laid off as many as 300 members of staff from its diagnostics and manufacturing division based out of Flanders, New Jersey.
The medical device giant has announced a series of FDA clearances recently, including the MAGNETOM Cima.X MRI scanner in January 2024, and the MAMMOMAT B.brilliant mammography system in April 2024.





