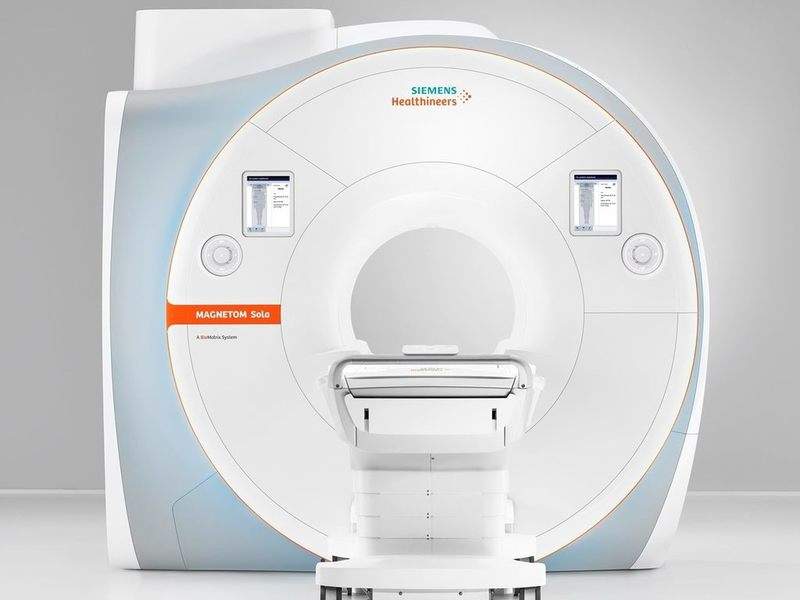
Siemens Healthineers has secured clearance from the US Food and Drug Administration (FDA) for its Magnetom Sola MRI scanner with 1.5 Tesla.
The new MRI is part of the company’s strategy to boost precision medicine. It features BioMatrix technology to address various anatomical, physiological, set up and performance differences.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Siemens noted that the technology is intended to enhance productivity and reduce rescans in order to enhance efficiency and patient satisfaction.
Magnetom Sola can be used for a range of routine and complex MRI exams. The MRI scanner is said to help in expediting workflow and delivering consistent results across all patient types.
BioMatrix Sensors are embedded in the device to save setup time and offer correct exam strategy.
In addition, Respiratory Sensors in the patient table are intended to avoid the need for navigators with respiratory-triggered sequences.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataA new in-bore camera system called Kinetic Sensor allows for visual monitoring of the patient’s face, while BioMatrix Tuners leverage distortion-mitigating software and hardware to improve the quality and reproducibility of head, neck and spine imaging.
Furthermore, the MRI scanner’s BioMatrix Interfaces uses artificial intelligence and body models to accelerate patient positioning and provide consistent, reproducible results.
Siemens Healthineers North America Magnetic Resonance vice-president Jane Kilkenney said: “With the Magnetom Sola, we provide the 1.5T market with the latest MRI advancements designed to overcome the challenges of imaging diverse patient populations.
“By delivering unmatched levels of automation and personalisation that address patient and user variability, we enable customers to achieve high-quality imaging – consistently and efficiently – across all patients and procedures.”
Magnetom Sola enables one-step coverage of larger body regions with a 50x50x50cm field of view. The scanner is available with lightweight, anatomy-adaptive coils to offer better comfort for patients.
The device also comes with new software for quick scanning while maintaining the quality of the images.
A specific variant of Magnetom Sola for cardiovascular scanning is available with relevant hardware and software such as Compressed Sensing Cardiac Cine, MyoMaps and the Cardiac Dot engine.



