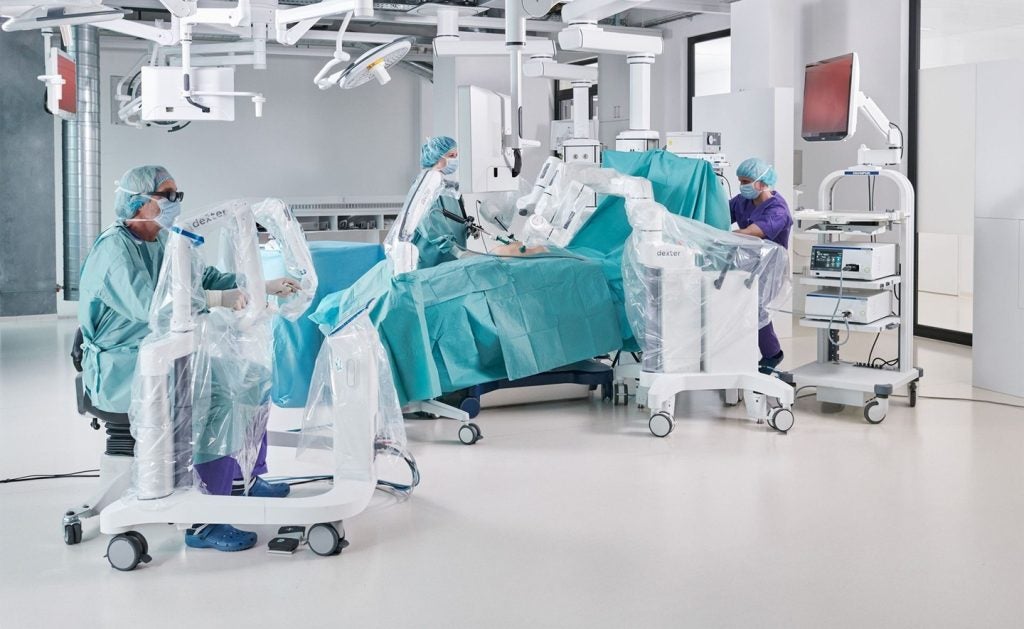
Illumina is expanding access to its oncology range of in vitro diagnostic (IVD) tests, as the company's TruSight Oncology (TSO) Comprehensive IVD is now covered under Medicare.
This coverage also extends to most commercial health plans in the US.
Preceding the US announcement, Illumina also announced that TSO Comprehensive has been approved by Japan’s Ministry of Health, Labour and Welfare (MHLW).
TSO uses next-generation sequencing (NGS) to detect variants in more than 500 genes using nucleic acids extracted from tumour tissue samples from cancer patients with solid malignant neoplasms to increase the likelihood that immuno-oncology or clinically actionable biomarkers will be identified.
Illumina’s chief commercial officer Everett Cunningham said: “With our growing portfolio of distributable clinical solutions, we are unlocking the next new standard of care for clinicians and their patients.”
As part of a 2023 partnership with Pillar Bioscience, Illumina will offer the former’s oncoReveal CDx to patients as of mid-2025. Used in sync with Illumina’s MiSeq Dx system, oncoReveal detects genetic variations in 22 genes and is intended for previously diagnosed patients with solid tumours. As of last month, the kit is also covered under Medicare.
Pillar’s chief marketing officer Brian Wright commented: “With over 66 million people in the US covered by Medicare, reimbursement of oncoReveal CDx will help ensure that highly accurate, actionable, and reimbursable next-generation sequencing testing is available to clinical laboratories and biopharmaceutical companies.”
Illumina’s TSO Comprehensive test and two companion diagnostic (CDx) indications secured approval from the US Food and Drug Administration (FDA) last year.
The CDx indications are for establishing whether adults and paediatric patients will benefit from treatment with Bayer’s Vitrakvi (larotrectinib) when neurotrophic tyrosine receptor kinase (NTRK) gene fusions are detected in solid tumours. They also determine adult patients with locally advanced or metastatic rearranged during transfection (RET) fusion-positive non-small-cell lung cancer (NSCLC).
TSO Comprehensive is part of Illumina’s broader TSO product portfolio. The company previously announced plans to launch TruSight Oncology 500 v2, a new version of its flagship next-NGS cancer assay for comprehensive genomic profiling (CGP) of solid tumours, by mid-2025.
According to GlobalData, the global NGS test devices market is set to reach a valuation of $5.1bn by 2033. Alongside Illumina, other leaders in the space include Thermo Fisher Scientific and Agilent Technologies.