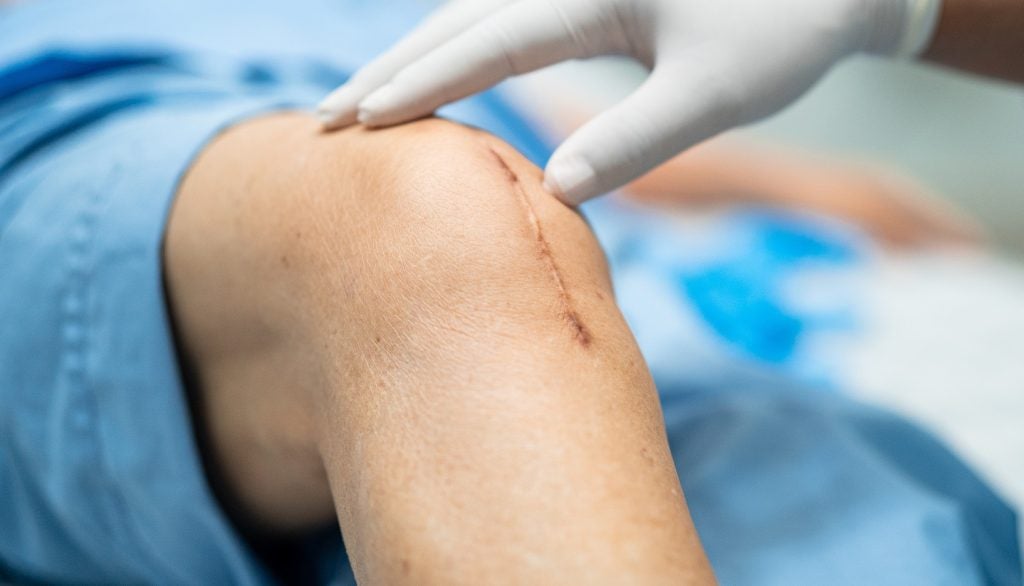
SAIL Fusion, a medical device company advancing the surgical treatment of sacroiliac joint dysfunction, has announced clearance from the US Food and Drug Administration (FDA) for its BowTie Sacroiliac Fusion System.
The BowTie Sacroiliac Fusion System is claimed to be the first sacroiliac (SI) fusion technology based on the AO Foundation's joint fusion principles.
It is the only device available that features both intra-articular and transfixing components, the company noted.
The minimally invasive surgery (MIS) BowTie technology, along with its posterior-inferior surgical approach, has been engineered to minimise tissue disruption.
It is designed to thoroughly prepare the joint for surgery and provide rigid fixation, which is essential for achieving true and robust arthrodesis.
The BowTie system's unique bowtie shape and the integration of transfixation and iliac screws are intended to maximise the rigidity of the fusion.
Furthermore, the device incorporates a porous structure and a roughened surface technology.
These features are specifically developed to support osteointegration.
SAIL Fusion president and CEO David Jansen said: “There hasn’t been meaningful differentiation in the SI fusion market for many years. By interviewing hundreds of surgeons, we were able to identify an unmet need for robust fixation built on proven AO principles.
“Our approach challenges the prevailing trend of lateral fixation that is more appropriate for stabilisation rather than true joint fusion. To achieve this, BowTie leverages decades of clinically validated arthrodesis methods that have been applied to nearly every other joint.”
The company plans to introduce the BowTie Sacroiliac Fusion System to a select group of surgeons initially. This move is in anticipation of a broader rollout in the subsequent months.
Jansen continued: “The BowTie device, with its trademark bowtie shape, was designed to help achieve the AO’s other significant joint fusion principle of rigidly fixating the joint to provide a biologic environment favourable for bone growth.
“We reinforced its rigidity by adding both integrated iliac and transfixation screws to address the complex biomechanics of the pelvis. We are pleased that BowTie’s multiplanar approach to fixating the joint also closely aligns with both the transfixation and the recently established intra-articular CPT codes. This is an exciting time for surgeons, and especially for patients struggling with chronic SI joint pain.”