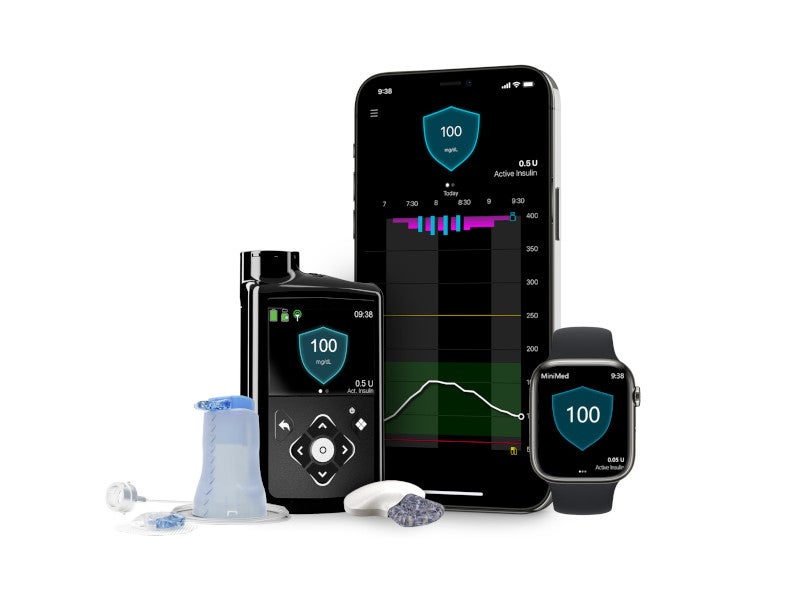
The MiniMed™ 780G system is an advanced insulin pump developed by Medtronic, a medical device company based in the US.
The system integrates the Guardian™ 4 sensor, eliminating the need for fingersticks when utilising SmartGuard™ technology.
It is the first system to feature meal detection technology, enabling automatic adjustments and corrections to sugar levels every five minutes for both basal (background) and bolus (mealtime) insulin requirements.
Moreover, it administers insulin to accommodate instances when users inadvertently forget to bolus or miscalculate the carbohydrate content of their meals.
Regulatory approvals
The US Food and Drug Administration (FDA) approved the system with the Guardian sensor in April 2023 for use in patients seven years old and above diagnosed with type 1 diabetes.
The FDA also approved the use of the MiniMed 780G system with the Simplera Sync™ sensor in April 2025.
In July 2025, the device secured the European CE Mark for expanded indications in insulin-requiring people with diabetes, individuals aged two years and older, and pregnant women.
Medtronic secured FDA clearance for the use of MiniMed 780G in adults (18-plus) with type 2 diabetes that requires insulin in September 2025, making it the only automated insulin delivery system with Meal Detection™ technology for this group.
Separately, the FDA approved the SmartGuard algorithm as an interoperable automated glycaemic controller, enabling the integration of Abbott’s Instinct sensor with MiniMed 780G system for type 1 diabetes.
The company began the commercial launch of the MiniMed 780G system paired with the Instinct sensor in the US in December 2025.
MiniMed 780G system details and design
The MiniMed 780G is an advanced hybrid closed-loop system with SmartGuard automation for self-adjusting basal delivery with auto-correction dosing. The system includes the Guardian continuous glucose monitor (CGM), MiniMed Mio™ advance infusion set and an insulin pump.
The Guardian sensor monitors the patient’s glucose levels 288 times a day with readings taken every five minutes. It then seamlessly transmits the data to the insulin pump, providing patients with real-time visibility into the glucose levels and their trajectory.
The waterproof insulin pump makes automatic adjustments and corrections to the insulin delivery and provides adjusted high or low concentrations of insulin to the patients based on the inputs received from the CGM every five minutes.
The MiniMed Mio advance infusion set is easy, fast and hassle-free with a pre-loaded inserter, facilitating quicker and easier set changes with fewer steps. The design allows for one-handed insertion, enabling effortless placement of the infusion set even in difficult-to-reach areas.
The system can use rapid-acting U100 insulin (Humalog® and NovoRapid®) prescribed by a healthcare professional.
Mode of operation
The MiniMed 780G system can be used in two different modes: Manual and SmartGuard.
Manual mode allows the usage of the pump with or without a CGM in a traditional way, as with the previous pump systems from Medtronic. The basal rates are pre-programmed in this mode.
On the other hand, SmartGuard mode is controlled by a SmartGuard algorithm, which automatically adjusts basal insulin every five minutes based on sensor glucose (SG) readings.
It can also automatically deliver a correction bolus to help correct a high SG reading. CGM is required in this mode.
MiniMed 780G system features
Designed to be accurate, comfortable and easy to use, the Guardian Sensor 4 measures glucose levels in the interstitial fluid and provides real-time glucose readings to the system.
The MiniMed 780G system allows for personalised settings to meet individual needs by adapting to the user’s insulin needs, considering factors such as insulin sensitivity, carbohydrate count and previous glucose patterns.
The system is compatible with the MiniMed Mobile App, which can be installed on a compatible smartphone. The app allows users to view their glucose data, insulin delivery history and system alerts conveniently on their mobile devices.
The MiniMed 780G insulin pump system is designed to withstand temperature conditions ranging from -4°F (-20°C) to 122°F (50°C).
The pump has 35 days of memory history.
Clinical trials with MiniMed 780G system
The results from the 90-day US pivotal trial of the MiniMed 780G system demonstrated positive outcomes in terms of time in range (TIR) and time below range (TBR), indicating improved glucose control for users.
TIR refers to the percentage of time when blood sugar levels were within the target range of 70mg per decilitre (dL)–180mg/dL, while TBR is defined as less than 70mg/dL.
The MiniMed 780G system achieved all study endpoints and exhibited substantial user satisfaction throughout the studies.
It includes a default target of 100mg/dL (with the alternative option of 120mg/dL), customisable insulin action time ranging from two to eight hours, and automatic corrections provided every five minutes.
The trial, examining adults and adolescents aged 14–75 years, effectively achieved its safety and glycaemic objectives, with no instances of severe adverse events reported.
According to the trial data, users of the MiniMed 780G system experienced a 75% overall TIR.
The overall TBR was reported to be 1.8%. Autocorrection comprised 22% of the total bolus insulin administered.
The outcomes also revealed that participants were engaged with SmartGuard (closed loop) functionality for 95% of the time. The average SG was 148mg/dL overall, and 144mg/dL when set at the default target of 100mg/dL.
The system’s performance was particularly notable during night-time, with an overnight TIR of 82% and an overnight TBR of 1.5%.
The results highlight the system’s ability to provide enhanced protection and glucose control during night-time.
The results from the first-of-its-kind Medtronic ADAPT study demonstrated a 27.6% increase in TIR and a 1.4% reduction in blood glucose levels among participants using the MiniMed 780G system compared to the standard of care (multiple daily injections (MDI) + CGM) at six months in type 1 diabetics.